Economic impact of switching from partially combined vaccine "Pentaxim® and hepatitis B" to fully combined vaccine "Hexaxim®" in the Malaysian National Immunization Program
- PMID: 34986870
- PMCID: PMC8734051
- DOI: 10.1186/s12913-021-07428-7
Economic impact of switching from partially combined vaccine "Pentaxim® and hepatitis B" to fully combined vaccine "Hexaxim®" in the Malaysian National Immunization Program
Abstract
Background: The decision to implement new vaccines should be supported by public health and economic evaluations. Therefore, this study was primarily designed to evaluate the economic impact of switching from partially combined vaccine (Pentaxim® plus hepatitis B) to fully combined vaccine (Hexaxim®) in the Malaysian National Immunization Program (NIP) and to investigate healthcare professionals (HCPs)' and parents'/caregivers' perceptions.
Methods: In this economic evaluation study, 22 primary healthcare centers were randomly selected in Malaysia between December 2019 and July 2020. The baseline immunization schedule includes switching from Pentaxim® (four doses) and hepatitis B (three doses) to Hexaxim® (four doses), whereas the alternative scheme includes switching from Pentaxim® (four doses) and hepatitis B (three doses) to Hexaxim® (four doses) and hepatitis B (one dose) administered at birth. Direct medical costs were extracted using a costing questionnaire and an observational time and motion chart. Direct non-medical (cost for transportation) and indirect costs (loss of productivity) were derived from parents'/caregivers' questionnaire. Also, HCPs' and parent's/caregivers' perceptions were investigated using structured questionnaires.
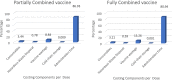
Results: The cost per dose of Pentaxim® plus hepatitis B vs. Hexaxim® for the baseline scheme was Malaysian ringgit (RM) 31.90 (7.7 United States dollar [USD]) vs. 17.10 (4.1 USD) for direct medical cost, RM 54.40 (13.1 USD) vs. RM 27.20 (6.6 USD) for direct non-medical cost, RM 221.33 (53.3 USD) vs. RM 110.66 (26.7 USD) for indirect cost, and RM 307.63 (74.2 USD) vs. RM 155.00 (37.4 USD) for societal (total) cost. A similar trend was observed for the alternative scheme. Compared with Pentaxim® plus hepatitis B, total cost savings per dose of Hexaxim® were RM 137.20 (33.1 USD) and RM 104.70 (25.2 USD) in the baseline and alternative scheme, respectively. Eighty-four percent of physicians and 95% of nurses supported the use of Hexaxim® in the NIP. The majority of parents/caregivers had a positive perception regarding Hexaxim® vaccine in various aspects.
Conclusions: Incorporation of Hexaxim® within Malaysian NIP is highly recommended because the use of Hexaxim® has demonstrated substantial direct and indirect cost savings for healthcare providers and parents/caregivers with a high percentage of positive perceptions, compared with Pentaxim® plus hepatitis B.
Trial registration: Not applicable.
Keywords: Economic impact; Hexaxim; Malaysia national immunization program; Pentaxim.
© 2022. The Author(s).
Conflict of interest statement
All author involved in these studies received grants from Sanofi-Aventis (Malaysia) Sdn Bhd through their respective institutions for the conduct of this study but did not receive any direct payment from Sanofi-Aventis (Malaysia) Sdn Bhd in this regard. They may have received reimbursement for expenses of conference attendance for the presentation of data from these studies.
Figures
Similar articles
-
Review of a new fully liquid, hexavalent vaccine: Hexaxim.Expert Opin Biol Ther. 2013 Apr;13(4):575-93. doi: 10.1517/14712598.2013.774368. Epub 2013 Feb 27. Expert Opin Biol Ther. 2013. PMID: 23441818 Review.
-
Parents' and healthcare professionals' perception toward the introduction of a new fully liquid hexavalent vaccine in the Malaysian national immunization program: a cross-sectional study instrument development and its application.Front Immunol. 2023 Apr 27;14:1052450. doi: 10.3389/fimmu.2023.1052450. eCollection 2023. Front Immunol. 2023. PMID: 37180162 Free PMC article.
-
Antibody persistence after a primary series of a new DTaP-IPV-Hep B-PRP-T combined vaccine or separate DTaP-IPV//PRP-T and hepatitis B vaccines at 2, 4, and 6 months of age and the effect of a subsequent DTaP-IPV//PRP-T booster vaccination at 18 months of age in healthy Argentinean infants.Pediatr Infect Dis J. 2012 Jan;31(1):e24-30. doi: 10.1097/INF.0b013e318242460a. Pediatr Infect Dis J. 2012. PMID: 22157567
-
DTaP-IPV-Hep B-Hib vaccine (Hexaxim®) : a review of its use in primary and booster vaccination.Paediatr Drugs. 2013 Feb;15(1):59-70. doi: 10.1007/s40272-013-0007-7. Paediatr Drugs. 2013. PMID: 23338932 Review.
-
Cost-minimization analysis of DTaP-IPV-Hib combination vaccine in China: A nationwide cross-sectional study.J Med Virol. 2023 Jan;95(1):e28358. doi: 10.1002/jmv.28358. J Med Virol. 2023. PMID: 36448181
Cited by
-
Immunogenicity and Safety of Childhood Combination Vaccines: A Systematic Review and Meta-Analysis.Vaccines (Basel). 2022 Mar 18;10(3):472. doi: 10.3390/vaccines10030472. Vaccines (Basel). 2022. PMID: 35335107 Free PMC article. Review.
-
Acceptance and willingness to pay for DTaP-HBV-IPV-Hib hexavalent vaccine among parents: A cross-sectional survey in China.Hum Vaccin Immunother. 2024 Dec 31;20(1):2333098. doi: 10.1080/21645515.2024.2333098. Epub 2024 Apr 15. Hum Vaccin Immunother. 2024. PMID: 38619056 Free PMC article.
-
New Vaccine Platforms-Novel Dimensions of Economic and Societal Value and Their Measurement.Vaccines (Basel). 2024 Feb 24;12(3):234. doi: 10.3390/vaccines12030234. Vaccines (Basel). 2024. PMID: 38543868 Free PMC article.
-
Methods used to account for caregivers' sex and gender within studies examining the financial burden of caregivers of children and adolescents : Results from a scoping review.Clinicoecon Outcomes Res. 2024 Jan 26;16:35-53. doi: 10.2147/CEOR.S443077. eCollection 2024. Clinicoecon Outcomes Res. 2024. PMID: 38298908 Free PMC article.
-
Estimating the Total Societal Cost of a Hexavalent Vaccine versus a Pentavalent Vaccine with Hepatitis B in South Korea.Vaccines (Basel). 2023 May 15;11(5):984. doi: 10.3390/vaccines11050984. Vaccines (Basel). 2023. PMID: 37243088 Free PMC article.
References
-
- Hall SN. The design and analysis of pediatric vaccine formularies: theory and practice [online] [PhD dissertation] Champaign: University of Illinois; 2006.
-
- Reportlinker . A white paper to understand the market structure of pediatric pertussis combination vaccines. 2020.
-
- Reinert P, Boucher J, Pines E, Leroux MC, Hoffenbach A, Salomon H, et al. Primary or booster immunization with DTaP–IPV vaccine administered either in combination or in association with Haemophilus influenzae type b vaccine (act-Hib): a large-scale safety study. Paris: Programs and Abstracts of the 15th Annual Meeting of the European Society for Paediatric Infectious Diseases; 1997.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical