A systematic review of outcomes in COVID-19 patients treated with western medicine in combination with traditional Chinese medicine versus western medicine alone
- PMID: 34986905
- PMCID: PMC8795778
- DOI: 10.1017/erm.2021.35
A systematic review of outcomes in COVID-19 patients treated with western medicine in combination with traditional Chinese medicine versus western medicine alone
Abstract
Background: Since the outbreak of coronavirus disease 2019 (COVID-19) in late 2019, it has evolved into a global pandemic that has become a substantial public health concern. COVID-19 is still causing a large number of deaths in several countries around the world because of the lack of effective treatment.
Aim: To systematically compare the outcomes of COVID-19 patients treated with integrated Chinese with western (ICW) medicine versus western medicine (WM) alone by pooling the data of published literature, and to determine if ICW treatment of COVID-19 patients has better clinical outcomes.
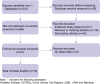
Methods: We searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), China Clinical Trial Registry, Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI) and Wanfang databases using keywords related to COVID-19, traditional Chinese medicine (TCM) and treatment effect. The search deadline was until 10 February 2021. All randomised controlled (RC) and non-randomised controlled (NRC) clinical trials of the ICW or WM treatment of COVID-19 patients were included. We analysed the effective rate, cure rate, exacerbation rate, turning negative rate of viral nucleic acid, remission rate and remission time of symptoms such as fever, cough, feebleness and chest computed tomography (CT) and the number of white blood cells (WBCs) and lymphocytes (LYM) of the COVID-19 patients. For qualitative and quantitative data, the ratio risk (RR) and weighted mean difference (WMD) were used as the indexes of the statistical analysis, respectively. RevMan 5.4 was used to perform meta-analyses and forest plots with the fixed-effects and random-effects models. Cochrane risk of bias tool (RoB 2.0) was used to assess the risk of bias in the included RC trials, whereas risk of bias in non-randomised studies of interventions was used to assess the risk of bias in NRC trials.
Results: This research includes 16 studies with 1645 valid confirmed COVID-19 patients, among which 895 patients of the experimental group received ICW treatment whereas 750 patients of the control group received WM treatment. The outcomes were assessed in three aspects, that is, overall indicator, symptoms indicator and blood indicator, respectively, and the results showed that the ICW group had better treatment outcomes compared with the WM. Among the overall indicators, the ICW group displayed a higher effective rate (RR = 1.24, 95% confidence interval (CI): 1.16-1.33), clinical cure rate (RR = 1.27, 95% CI: 1.03-1.56) and lower exacerbation rate (RR = 0.36, 95% CI: 0.25-0.52), but no statistical difference was observed in the turning negative rate of viral nucleic acid (RR = 1.20, 95% CI: 0.78-1.85). Among the symptom indicators, the ICW group had a higher fever remission rate (RR = 1.24, 95% CI: 1.09-1.42), less fever remission time (WMD = -1.49, 95% CI: -1.85 to -1.12), a higher cough remission rate (RR = 1.38, 95% CI: 1.10-1.73) and a feebleness remission rate (RR = 1.45, 95% CI: 1.18-1.77), less cough remission time (WMD = -1.61, 95% CI: -2.35 to -0.87) and feebleness remission time (WMD = -1.50, 95% CI: -2.38 to -0.61) and better improvement in chest CT (RR = 1.19, 95% CI: 1.11-1.28). For blood indicator, the number of WBCs in the blood of patients of ICW group rebounded significantly (WMD = 0.35, 95% CI: 0.16-0.54), and the recovery of LYM in the blood was more obvious (WMD = 0.23, 95% CI: 0.06-0.40).
Conclusion: The results of this study show that the outcomes in COVID-19 patients treated by the ICW is better than those treated by the WM treatment alone, suggesting that WM and TCM can be complementary in the treatment of COVID-19.
Keywords: Coronavirus disease 2019 (COVID-19); integrated Chinese with western (ICW); meta-analysis; traditional Chinese medicine (TCM); western medicine (WM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















References
-
- World Health Organization. Coronavirus Disease (COVID-19) Dashboard. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio....
-
- The State Council Information Office of the People's Republic of China. Fighting COVID-19: China in Action. June 2020. Available at http://www.scio.gov.cn/zfbps/32832/Document/1681809/1681809.htm.
-
- Zhou F et al. (2021) Efficacy and safety of Chinese herbal decoction combined with western medicine in treatment of COVID-19: a meta-analysis. Journal of Medical 37, 564–568.
-
- Liu L et al. (2021) A review and meta-analysis of clinical efficacy and safety of the integrative medicine on COVID-19. Clinical Journal of Chinese Medicine 13, 24–30.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

