The Yin and Yang of ERBB4: Tumor Suppressor and Oncoprotein
- PMID: 34987087
- PMCID: PMC11060329
- DOI: 10.1124/pharmrev.121.000381
The Yin and Yang of ERBB4: Tumor Suppressor and Oncoprotein
Abstract
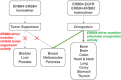
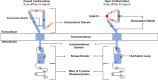
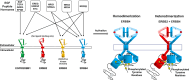
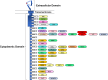
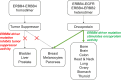
ERBB4 (HER4) is a member of the ERBB family of receptor tyrosine kinases, a family that includes the epidermal growth factor receptor (EGFR/ERBB1/HER1), ERBB2 (Neu/HER2), and ERBB3 (HER3). EGFR and ERBB2 are oncoproteins and validated targets for therapeutic intervention in a variety of solid tumors. In contrast, the role that ERBB4 plays in human malignancies is ambiguous. Thus, here we review the literature regarding ERBB4 function in human malignancies. We review the mechanisms of ERBB4 signaling with an emphasis on mechanisms of signaling specificity. In the context of this signaling specificity, we discuss the hypothesis that ERBB4 appears to function as a tumor suppressor protein and as an oncoprotein. Next, we review the literature that describes the role of ERBB4 in tumors of the bladder, liver, prostate, brain, colon, stomach, lung, bone, ovary, thyroid, hematopoietic tissues, pancreas, breast, skin, head, and neck. Whenever possible, we discuss the possibility that ERBB4 mutants function as biomarkers in these tumors. Finally, we discuss the potential roles of ERBB4 mutants in the staging of human tumors and how ERBB4 function may dictate the treatment of human tumors. SIGNIFICANCE STATEMENT: This articles reviews ERBB4 function in the context of the mechanistic model that ERBB4 homodimers function as tumor suppressors, whereas ERBB4-EGFR or ERBB4-ERBB2 heterodimers act as oncogenes. Thus, this review serves as a mechanistic framework for clinicians and scientists to consider the role of ERBB4 and ERBB4 mutants in staging and treating human tumors.
Copyright © 2022 by The Author(s).
Figures


References
-
- Ahammad I, Sarker MRI, Khan AM, Islam S, Hossain M (2020) Virtual screening to identify novel inhibitors of pan ERBB family of proteins from natural products with known anti-tumorigenic properties. Int J Pept Res Ther 26:1923–1938.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

