Sex Differences in Opioid and Psychostimulant Craving and Relapse: A Critical Review
- PMID: 34987089
- PMCID: PMC11060335
- DOI: 10.1124/pharmrev.121.000367
Sex Differences in Opioid and Psychostimulant Craving and Relapse: A Critical Review
Abstract
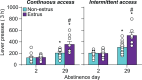
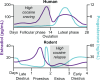
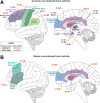
A widely held dogma in the preclinical addiction field is that females are more vulnerable than males to drug craving and relapse. Here, we first review clinical studies on sex differences in psychostimulant and opioid craving and relapse. Next, we review preclinical studies on sex differences in psychostimulant and opioid reinstatement of drug seeking after extinction of drug self-administration, and incubation of drug craving (time-dependent increase in drug seeking during abstinence). We also discuss ovarian hormones' role in relapse and craving in humans and animal models and speculate on brain mechanisms underlying their role in cocaine craving and relapse in rodent models. Finally, we discuss imaging studies on brain responses to cocaine cues and stress in men and women.The results of the clinical studies reviewed do not appear to support the notion that women are more vulnerable to psychostimulant and opioid craving and relapse. However, this conclusion is tentative because most of the studies reviewed were correlational, not sufficiently powered, and not a priori designed to detect sex differences. Additionally, imaging studies suggest sex differences in brain responses to cocaine cues and stress. The results of the preclinical studies reviewed provide evidence for sex differences in stress-induced reinstatement and incubation of cocaine craving but not cue- or cocaine-induced reinstatement of cocaine seeking. These sex differences are modulated in part by ovarian hormones. In contrast, the available data do not support the notion of sex differences in craving and relapse/reinstatement for methamphetamine or opioids in rodent models. SIGNIFICANCE STATEMENT: This systematic review summarizes clinical and preclinical studies on sex differences in psychostimulant and opioid craving and relapse. Results of the clinical studies reviewed do not appear to support the notion that women are more vulnerable to psychostimulant and opioid craving and relapse. Results of preclinical studies reviewed provide evidence for sex differences in reinstatement and incubation of cocaine seeking but not for reinstatement or incubation of methamphetamine or opioid seeking.
U.S. Government work not protected by U.S. copyright.
Figures


References
-
- Adelson M, Linzy S, Peles E (2018) Characteristics and outcome of male and female methadone maintenance patients: MMT in Tel Aviv and Las Vegas. Subst Use Misuse 53:230–238. - PubMed
-
- Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22:413–421. - PubMed

