Decarboxylative cross-nucleophile coupling via ligand-to-metal charge transfer photoexcitation of Cu(II) carboxylates
- PMID: 34987174
- PMCID: PMC8820273
- DOI: 10.1038/s41557-021-00834-8
Decarboxylative cross-nucleophile coupling via ligand-to-metal charge transfer photoexcitation of Cu(II) carboxylates
Abstract
Reactions that enable carbon-nitrogen, carbon-oxygen and carbon-carbon bond formation lie at the heart of synthetic chemistry. However, substrate prefunctionalization is often needed to effect such transformations without forcing reaction conditions. The development of direct coupling methods for abundant feedstock chemicals is therefore highly desirable for the rapid construction of complex molecular scaffolds. Here we report a copper-mediated, net-oxidative decarboxylative coupling of carboxylic acids with diverse nucleophiles under visible-light irradiation. Preliminary mechanistic studies suggest that the relevant chromophore in this reaction is a Cu(II) carboxylate species assembled in situ. We propose that visible-light excitation to a ligand-to-metal charge transfer (LMCT) state results in a radical decarboxylation process that initiates the oxidative cross-coupling. The reaction is applicable to a wide variety of coupling partners, including complex drug molecules, suggesting that this strategy for cross-nucleophile coupling would facilitate rapid compound library synthesis for the discovery of new pharmaceutical agents.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interest declaration
NJB, MWB, JWT and SWB are employees and shareholders of Pfizer, Inc. The remaining authors declare no competing interests.
Figures
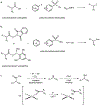
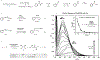
References
-
- Xuan J, Zhang Z-G & Xiao W-J. Visible-light-induced decarboxylative functionalization of carboxylic acids and their derivatives. Angew. Chem. Int. Ed 54, 15632–15641 (2015). - PubMed
-
- Rodríguez N & Goossen LJ Decarboxylative coupling reactions: A modern strategy for C–C-bond formation. Chem. Soc. Rev 40, 5030–5048 (2011). - PubMed
-
- Zeng Z, Feceu A, Sivendran N & Gooßen LJ Decarboxylation - initiated intermolecular carbon–heteroatom bond formation. Adv. Synth. Catal 363, 2678–2722 (2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

