Lipid Metabolism Influence on Neurodegenerative Disease Progression: Is the Vehicle as Important as the Cargo?
- PMID: 34987360
- PMCID: PMC8721228
- DOI: 10.3389/fnmol.2021.788695
Lipid Metabolism Influence on Neurodegenerative Disease Progression: Is the Vehicle as Important as the Cargo?
Abstract
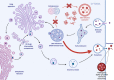
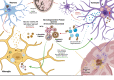
Many neurodegenerative diseases are characterized by abnormal protein aggregates, including the two most common neurodegenerative diseases Alzheimer's disease (AD) and Parkinson's disease (PD). In the global search to prevent and treat diseases, most research has been focused on the early stages of the diseases, including how these pathogenic protein aggregates are initially formed. We argue, however, that an equally important aspect of disease etiology is the characteristic spread of protein aggregates throughout the nervous system, a key process in disease progression. Growing evidence suggests that both alterations in lipid metabolism and dysregulation of extracellular vesicles (EVs) accelerate the spread of protein aggregation and progression of neurodegeneration, both in neurons and potentially in surrounding glia. We will review how these two pathways are intertwined and accelerate the progression of AD and PD. Understanding how lipid metabolism, EV biogenesis, and EV uptake regulate the spread of pathogenic protein aggregation could reveal novel therapeutic targets to slow or halt neurodegenerative disease progression.
Keywords: Alzheimer’s disease; Parkinson’s disease; ceramide; extracellular vesicle; glia; glucocerebrosidase (GBA); lipid metabolism; protein aggregation and propagation.
Copyright © 2021 Estes, Lin, Khera and Davis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ahmed Z., Cooper J., Murray T. K., Garn K., Mcnaughton E., Clarke H., et al. (2014). A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 127, 667–683. 10.1007/s00401-014-1254-6 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

