α-Mangostin Alleviated HIF-1α-Mediated Angiogenesis in Rats With Adjuvant-Induced Arthritis by Suppressing Aerobic Glycolysis
- PMID: 34987400
- PMCID: PMC8721667
- DOI: 10.3389/fphar.2021.785586
α-Mangostin Alleviated HIF-1α-Mediated Angiogenesis in Rats With Adjuvant-Induced Arthritis by Suppressing Aerobic Glycolysis
Abstract
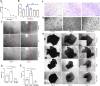
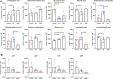
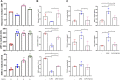
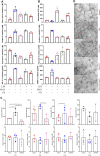
A previously validated anti-rheumatic compound α-mangostin (MAN) shows significant metabolism regulatory effects. The current study aimed to clarify whether this property contributed to its inhibition on synovial angiogenesis. Male wistar rats with adjuvant-induced arthritis (AIA) were orally treated by MAN for 32 days. Afterwards, biochemical parameters and cytokines in plasma were determined by corresponding kits, and glycometabolism-related metabolites were further accurately quantified by LC-MS method. Anti-angiogenic effects of MAN were preliminarily assessed by joints based-immunohistochemical examination and matrigel plug assay. Obtained results were then validated by experiments in vitro. AIA-caused increase in circulating transforming growth factor beta, interleukin 6, hypoxia inducible factor-1 alpha (HIF-1α) and vascular endothelial growth factor (VEGF) in blood and local HIF-1α/VEGF expression in joints was abrogated by MAN treatment, and pannus formation within matrigel plugs implanted in AIA rats was inhibited too. Scratch and transwell assays revealed the inhibitory effects of MAN on human umbilical vein endothelial cells (HUVECs) migration. Furthermore, MAN inhibited tubule formation capability of HUVECs and growth potential of rat arterial ring-derived endothelial cells in vitro. Meanwhile, MAN eased oxidative stress, and altered glucose metabolism in vivo. Glycolysis-related metabolites including glucose 6-phosphate, fructose 6-phosphate, 3-phosphoglyceric acid and phosphoenolpyruvic acid in AIA rats were decreased by MAN, while the impaired pyruvate-synthesizing capability of lactate dehydrogenase (LDH) was recovered. Consistently, MAN restored lipopolysaccharide-elicited changes on levels of glucose and LDH in HUVECs culture system, and exerted similar effects with LDH inhibitor stiripentol on glycometabolism and VEGF production as well as tubule formation capability of HUVECs. These evidences show that MAN treatment inhibited aerobic glycolysis in AIA rats, which consequently eased inflammation-related hypoxia, and hampered pathological neovascularization.
Keywords: glycometabolism; hypoxia inducible factor-1α (HIF-1α); metabolism reprogramming; oxidative stress MAN suppressed glycolysis-related angiogenesis; rheumatoid arthritis (RA).
Copyright © 2021 Jiang, Ji, Cheng, Gu, Wang, Li, Zuo and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Resveratrol-induced SIRT1 activation inhibits glycolysis-fueled angiogenesis under rheumatoid arthritis conditions independent of HIF-1α.Inflamm Res. 2023 May;72(5):1021-1035. doi: 10.1007/s00011-023-01728-w. Epub 2023 Apr 4. Inflamm Res. 2023. PMID: 37016140
-
α-Mangostin Alleviated Inflammation in Rats With Adjuvant-Induced Arthritis by Disrupting Adipocytes-Mediated Metabolism-Immune Feedback.Front Pharmacol. 2021 Jul 7;12:692806. doi: 10.3389/fphar.2021.692806. eCollection 2021. Front Pharmacol. 2021. PMID: 34305602 Free PMC article.
-
AMSP-30 m as a novel HIF-1α inhibitor attenuates the development and severity of adjuvant-induced arthritis in rats: Impacts on synovial apoptosis, synovial angiogenesis and sonic hedgehog signaling pathway.Int Immunopharmacol. 2022 Feb;103:108467. doi: 10.1016/j.intimp.2021.108467. Epub 2021 Dec 20. Int Immunopharmacol. 2022. PMID: 34933161
-
Diosgenin in Dioscorea spongiosa Suppresses Glycolysis-Driven Angiogenesis as a ROCK1 Inhibitor.J Agric Food Chem. 2025 Apr 30;73(17):10214-10229. doi: 10.1021/acs.jafc.4c11678. Epub 2025 Apr 19. J Agric Food Chem. 2025. PMID: 40252032
-
Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/β-catenin pathway in gliomas.Rev Neurosci. 2018 Jan 26;29(1):71-91. doi: 10.1515/revneuro-2017-0032. Rev Neurosci. 2018. PMID: 28822229 Review.
Cited by
-
Engineering 3D-BMSC exosome-based hydrogels that collaboratively regulate bone microenvironment and promote osteogenesis for enhanced cell-free bone regeneration.Mater Today Bio. 2025 May 20;32:101881. doi: 10.1016/j.mtbio.2025.101881. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40510837 Free PMC article.
-
Anti-inflammatory effects of 1,7-dihydroxy-3,4-dimethoxyxanthone through inhibition of M1-phenotype macrophages via arginine/mitochondrial axis.Immunol Res. 2024 Dec;72(6):1404-1416. doi: 10.1007/s12026-024-09538-w. Epub 2024 Sep 30. Immunol Res. 2024. PMID: 39349673
-
Inhibition mechanism investigation of quercetagetin as a potential tyrosinase inhibitor.Front Chem. 2024 Jun 3;12:1411801. doi: 10.3389/fchem.2024.1411801. eCollection 2024. Front Chem. 2024. PMID: 38894729 Free PMC article.
-
Resveratrol-induced SIRT1 activation inhibits glycolysis-fueled angiogenesis under rheumatoid arthritis conditions independent of HIF-1α.Inflamm Res. 2023 May;72(5):1021-1035. doi: 10.1007/s00011-023-01728-w. Epub 2023 Apr 4. Inflamm Res. 2023. PMID: 37016140
-
An Update on Stiripentol Mechanisms of Action: A Narrative Review.Adv Ther. 2024 Apr;41(4):1351-1371. doi: 10.1007/s12325-024-02813-0. Epub 2024 Mar 5. Adv Ther. 2024. PMID: 38443647 Free PMC article. Review.
References
-
- Connolly M., Marrelli A., Blades M., McCormick J., Maderna P., Godson C., et al. (2010). Acute Serum Amyloid A Induces Migration, Angiogenesis, and Inflammation in Synovial Cells In Vitro and in a Human Rheumatoid arthritis/SCID Mouse Chimera Model. J. Immunol. 184, 6427–6437. 10.4049/jimmunol.0902941 - DOI - PubMed
LinkOut - more resources
Full Text Sources

