mTORC1 Signaling Pathway Mediates Chronic Stress-Induced Synapse Loss in the Hippocampus
- PMID: 34987410
- PMCID: PMC8722735
- DOI: 10.3389/fphar.2021.801234
mTORC1 Signaling Pathway Mediates Chronic Stress-Induced Synapse Loss in the Hippocampus
Abstract
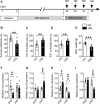
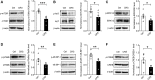
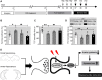
Background: The mechanistic target of rapamycin complex 1 (mTORC1) signaling has served as a promising target for therapeutic intervention of major depressive disorder (MDD), but the mTORC1 signaling underlying MDD has not been well elucidated. In the present study, we investigated whether mTORC1 signaling pathway mediates synapse loss induced by chronic stress in the hippocampus. Methods: Chronic restraint stress-induced depression-like behaviors were tested by behavior tests (sucrose preference test, forced swim test and tail suspension test). Synaptic proteins and alternations of phosphorylation levels of mTORC1 signaling-associated molecules were measured using Western blotting. In addition, mRNA changes of immediate early genes (IEGs) and glutamate receptors were measured by RT-PCR. Rapamycin was used to explore the role of mTORC1 signaling in the antidepressant effects of fluoxetine. Results: After successfully establishing the chronic restraint stress paradigm, we observed that the mRNA levels of some IEGs were significantly changed, indicating the activation of neurons and protein synthesis alterations. Then, there was a significant downregulation of glutamate receptors and postsynaptic density protein 95 at protein and mRNA levels. Additionally, synaptic fractionation assay revealed that chronic stress induced synapse loss in the dorsal and ventral hippocampus. Furthermore, these effects were associated with the mTORC1 signaling pathway-mediated protein synthesis, and subsequently the phosphorylation of associated downstream signaling targets was reduced after chronic stress. Finally, we found that intracerebroventricular infusion of rapamycin simulated depression-like behavior and also blocked the antidepressant effects of fluoxetine. Conclusion: Overall, our study suggests that mTORC1 signaling pathway plays a critical role in mediating synapse loss induced by chronic stress, and has part in the behavioral effects of antidepressant treatment.
Keywords: chronic restraint stress; depression; fluoxetine; mammalian target of rapamycin; postsynaptic density protein 95.
Copyright © 2021 Luo, Ye, Fang, Li, Xia, Liu, Lin, Huang, Zhu, Huang, Tan, Zhang, Liu, Zhou and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Admon R., Leykin D., Lubin G., Engert V., Andrews J., Pruessner J., et al. (2013). Stress-induced Reduction in Hippocampal Volume and Connectivity with the Ventromedial Prefrontal Cortex Are Related to Maladaptive Responses to Stressful Military Service. Hum. Brain Mapp. 34 (11), 2808–2816. 10.1002/hbm.22100 - DOI - PMC - PubMed
-
- Armbrecht E., Shah R., Poorman G. W., Luo L., Stephens J. M., Li B., et al. (2021). Economic and Humanistic Burden Associated with Depression and Anxiety Among Adults with Non-communicable Chronic Diseases (NCCDs) in the United States. J. Multidiscip Healthc. 14, 887–896. 10.2147/JMDH.S280200 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

