Fatal Tumour Lysis Syndrome Induced by Brigatinib in a Lung Adenocarcinoma Patient Treated With Sequential ALK Inhibitors: A Case Report
- PMID: 34987411
- PMCID: PMC8721166
- DOI: 10.3389/fphar.2021.809467
Fatal Tumour Lysis Syndrome Induced by Brigatinib in a Lung Adenocarcinoma Patient Treated With Sequential ALK Inhibitors: A Case Report
Abstract
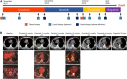
Tumour lysis syndrome (TLS) represents a group of fatal metabolic derangements resulting from the rapid breakdown of tumour cells. TLS typically occurs soon after the administration of chemotherapy in haematologic malignancies but is rarely observed in solid tumours. Here, we report a case of brigatinib-induced TLS after treatment with sequential anaplastic lymphoma kinase (ALK) inhibitors in a patient with advanced ALK-rearranged lung adenocarcinoma. The patient was treated sequentially with crizotinib, alectinib, and ensartinib. High-throughput molecular profiling after disease progression indicated that brigatinib may overcome ALK resistance mutations, so the patient was administered brigatinib as the fourth-line treatment. After 22 days of therapy, he developed oliguria, fever, and progressive dyspnoea. Clinical manifestations and laboratory findings met the diagnostic criteria for TLS. The significant decrease in the abundance of ALK mutations in plasma indicated a therapeutic response at the molecular level. Consequently, the diagnosis of brigatinib-induced TLS was established. To the best of our knowledge, this is the first case of TLS induced by sequential targeted therapy in non-small cell lung cancer. With the extensive application of sequential therapy with more potent next-generation targeted therapeutic drugs, special attention should be given to this rare but severe complication.
Keywords: acute kidney injury; brigatinib; case report; non-small cell lung cancer; targeted therapy; tumour lysis syndrome.
Copyright © 2021 Wang, Wang, Xue, Jia, Liu, Li, Li, Li, Wang, Bing, Cao, Cao and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial.Lancet Oncol. 2016 Dec;17(12):1683-1696. doi: 10.1016/S1470-2045(16)30392-8. Epub 2016 Nov 8. Lancet Oncol. 2016. PMID: 27836716 Clinical Trial.
-
ALTA-2: Phase II study of brigatinib in patients with ALK-positive, advanced non-small-cell lung cancer who progressed on alectinib or ceritinib.Future Oncol. 2021 May;17(14):1709-1719. doi: 10.2217/fon-2020-1119. Epub 2021 Feb 11. Future Oncol. 2021. PMID: 33569983
-
Intracranial remission with brigatinib rechallenge as fifth-line ALK inhibition therapy in a lung cancer patient.Anticancer Drugs. 2019 Nov;30(10):1058-1060. doi: 10.1097/CAD.0000000000000800. Anticancer Drugs. 2019. PMID: 31033499
-
Alectinib and Brigatinib: New Second-Generation ALK Inhibitors for the Treatment of Non-Small Cell Lung Cancer.J Adv Pract Oncol. 2018 Jan-Feb;9(1):94-101. Epub 2018 Jan 1. J Adv Pract Oncol. 2018. PMID: 30564472 Free PMC article. Review.
-
An evaluation of brigatinib as a promising treatment option for non-small cell lung cancer.Expert Opin Pharmacother. 2019 Sep;20(13):1551-1561. doi: 10.1080/14656566.2019.1643839. Epub 2019 Jul 22. Expert Opin Pharmacother. 2019. PMID: 31328968 Review.
Cited by
-
Nephrotoxicity of targeted therapy used to treat lung cancer.Front Immunol. 2024 Jul 4;15:1369118. doi: 10.3389/fimmu.2024.1369118. eCollection 2024. Front Immunol. 2024. PMID: 39026680 Free PMC article. Review.
-
Acute Kidney Injury Associated with Anticancer Therapies: Small Molecules and Targeted Therapies.Kidney360. 2024 Nov 1;5(11):1750-1762. doi: 10.34067/KID.0000000566. Epub 2024 Aug 26. Kidney360. 2024. PMID: 39186376 Free PMC article. Review.
-
A novel secondary ALK gene mutation which resistant to second-generation TKIs: a case report and literature review.Front Oncol. 2024 Aug 29;14:1430350. doi: 10.3389/fonc.2024.1430350. eCollection 2024. Front Oncol. 2024. PMID: 39267820 Free PMC article.
-
Afatinib-Induced Tumor Lysis Syndrome in Pulmonary Adenocarcinoma: A Case Report and Literature Review.Medicina (Kaunas). 2023 Dec 10;59(12):2144. doi: 10.3390/medicina59122144. Medicina (Kaunas). 2023. PMID: 38138247 Free PMC article. Review.
References
-
- Cairo M. S., Coiffier B., Reiter A., Younes A. (2010). Recommendations for the Evaluation of Risk and Prophylaxis of Tumour Lysis Syndrome (TLS) in Adults and Children with Malignant Diseases: An Expert TLS Panel Consensus. Br. J. Haematol. 149 (4), 578–586. 10.1111/j.1365-2141.2010.08143.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

