Tranexamic Acid for Acute Spontaneous Intracerebral Hemorrhage: A Meta-Analysis of Randomized Controlled Trials
- PMID: 34987465
- PMCID: PMC8720763
- DOI: 10.3389/fneur.2021.761185
Tranexamic Acid for Acute Spontaneous Intracerebral Hemorrhage: A Meta-Analysis of Randomized Controlled Trials
Abstract
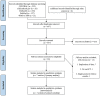
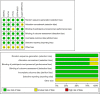
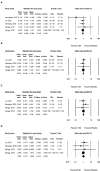
Background: The role of tranexamic acid (TXA) in preventing hematoma expansion (HE) in patients with acute spontaneous intracerebral hemorrhage (ICH) remains unclear. We aim to investigate the efficacy and safety of TXA in acute spontaneous ICH with a particular focus on subgroups. Methods: Randomized controlled trials (RCTs) were retrieved from CENTRAL, Clinicaltrials.gov, EMBASE, PubMed, and WHO ICTRP. The primary outcome measurement was HE. The secondary outcome measurements included 3-month poor functional outcome (PFO), 3-month mortality, and major thromboembolic events (MTE). We conducted subgroup analysis according to the CT markers of HE (standard-risk population and high-risk population) and the time from onset to randomization (>4.5 and ≤4.5 h). Results: We identified seven studies (representing five RCTs) involving 2,650 participants. Compared with placebo, TXA may reduce HE on subsequent imaging (odd ratio [OR] 0.825; 95% confidence interval [CI] 0.692-0.984; p = 0.033; I2 = 0%; GRADE: moderate certainty). TXA and placebo arms did not differ in the rates of 3-month PFO, 3-month mortality, and MTE. Subgroup analysis indicated that TXA reduced the risk of HE in the high-risk population with CT markers of HE (OR 0.646; 95% CI 0.503-0.829; p = 0.001; I2 = 0 %) and in patients who were treated within 4.5 h of symptom onset (OR 0.823; 95% CI 0.690-0.980; p = 0.029; I2 = 0%), but this protective effect was not observed in the standard-risk population and patients who were treated over 4.5 h of symptom onset. Conclusions: Tranexamic acid (TXA) may decrease the risk of HE in patients with acute spontaneous ICH. Importantly, the decreased risk was observed in patients who were treatable within 4.5 h and with a high risk of HE, but not in those who were treatable over 4.5 h and in standard-risk population. However, PFO or mortality at 3 months did not significantly differ between patients who received TXA and those who received placebo. TXA is safe for acute spontaneous ICH without increasing MTE.
Keywords: cerebral hemorrhage; hematoma; meta-analysis; randomized controlled trial; tranexamic acid.
Copyright © 2021 Guo, Guo, Li, Zhao, Bao, Yang, Zhang and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
-
- GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1736–88. 10.1016/S0140-6736(18)32203-7 - DOI - PMC - PubMed
-
- Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2032–60. 10.1161/STR.0000000000000069 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

