Disorders of Sex Development of Adrenal Origin
- PMID: 34987475
- PMCID: PMC8720965
- DOI: 10.3389/fendo.2021.770782
Disorders of Sex Development of Adrenal Origin
Abstract
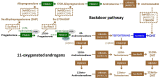
Disorders of Sex Development (DSD) are anomalies occurring in the process of fetal sexual differentiation that result in a discordance between the chromosomal sex and the sex of the gonads and/or the internal and/or external genitalia. Congenital disorders affecting adrenal function may be associated with DSD in both 46,XX and 46,XY individuals, but the pathogenic mechanisms differ. While in 46,XX cases, the adrenal steroidogenic disorder is responsible for the genital anomalies, in 46,XY patients DSD results from the associated testicular dysfunction. Primary adrenal insufficiency, characterized by a reduction in cortisol secretion and overproduction of ACTH, is the rule. In addition, patients may exhibit aldosterone deficiency leading to salt-wasting crises that may be life-threatening. The trophic effect of ACTH provokes congenital adrenal hyperplasia (CAH). Adrenal steroidogenic defects leading to 46,XX DSD are 21-hydroxylase deficiency, by far the most prevalent, and 11β-hydroxylase deficiency. Lipoid Congenital Adrenal Hyperplasia due to StAR defects, and cytochrome P450scc and P450c17 deficiencies cause DSD in 46,XY newborns. Mutations in SF1 may also result in combined adrenal and testicular failure leading to DSD in 46,XY individuals. Finally, impaired activities of 3βHSD2 or POR may lead to DSD in both 46,XX and 46,XY individuals. The pathophysiology, clinical presentation and management of the above-mentioned disorders are critically reviewed, with a special focus on the latest biomarkers and therapeutic development.
Keywords: DSD; adrenal insufficiency; aldosterone; congenital adrenal hyperplasia; cortisol; glucocorticoid; lipoid; mineralocorticoid.
Copyright © 2021 Finkielstain, Vieites, Bergadá and Rey.
Conflict of interest statement
GF is currently employed by Takeda Pharma S.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Freire AV, Ropelato MG, Rey RA. Ovaries and Testes. In: Kovacs CS, Deal C, editors. Maternal-Fetal and Neonatal Endocrinology, 1st ed. Boston, MA, USA: Academic Press-Elsevier; (2020). p. 625–41.
-
- Rey R, Josso N, Racine C. Sexual Differentiation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, editors. Endotext. South Dartmouth (MA), USA: MDText.com, Inc; (2020).
-
- Lee PA, Houk CP, Ahmed SF, Hughes IA. In Collaboration With the Participants in the International Consensus Conference on Intersex Organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus Statement on Management of Intersex Disorders. Pediatrics (2006) 118:e488–500. doi: 10.1542/peds.2006-0738 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

