Detection of Antibody Responses Against SARS-CoV-2 in Plasma and Saliva From Vaccinated and Infected Individuals
- PMID: 34987505
- PMCID: PMC8721203
- DOI: 10.3389/fimmu.2021.759688
Detection of Antibody Responses Against SARS-CoV-2 in Plasma and Saliva From Vaccinated and Infected Individuals
Abstract
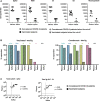
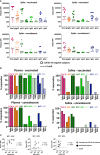
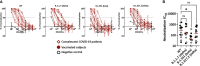
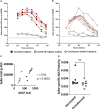
Antibodies (Abs) are essential for the host immune response against SARS-CoV-2, and all the vaccines developed so far have been designed to induce Abs targeting the SARS-CoV-2 spike. Many studies have examined Ab responses in the blood from vaccinated and infected individuals. However, since SARS-CoV-2 is a respiratory virus, it is also critical to understand the mucosal Ab responses at the sites of initial virus exposure. Here, we examined plasma versus saliva Ab responses in vaccinated and convalescent patients. Although saliva levels were significantly lower, a strong correlation was observed between plasma and saliva total Ig levels against all SARS-CoV-2 antigens tested. Virus-specific IgG1 responses predominated in both saliva and plasma, while a lower prevalence of IgM and IgA1 Abs was observed in saliva. Antiviral activities of plasma Abs were also studied. Neutralization titers against the initial WA1 (D614G), B.1.1.7 (alpha) and B.1.617.2 (delta) strains were similar but lower against the B.1.351 (beta) strain. Spike-specific antibody-dependent cellular phagocytosis (ADCP) activities were also detected and the levels correlated with spike-binding Ig titers. Interestingly, while neutralization and ADCP potencies of vaccinated and convalescent groups were comparable, enhanced complement deposition to spike-specific Abs was noted in vaccinated versus convalescent groups and corresponded with higher levels of IgG1 plus IgG3 among the vaccinated individuals. Altogether, this study demonstrates the detection of Ab responses after vaccination or infection in plasma and saliva that correlate significantly, although Ig isotypic differences were noted. The induced plasma Abs displayed Fab-mediated and Fc-dependent functions with comparable neutralization and ADCP potencies, but a greater capacity to activate complement was elicited upon vaccination.
Keywords: ADCP; COVID-19; SARS-CoV-2; antibody isotypes; complement fixation; neutralization; saliva; vaccination.
Copyright © 2021 Klingler, Lambert, Itri, Liu, Bandres, Enyindah-Asonye, Liu, Simon, Gleason, Kleiner, Chiu, Hung, Kowdle, Amanat, Lee, Zolla-Pazner, Upadhyay and Hioe.
Conflict of interest statement
The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and listed VS as co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Update of
-
Detection of Antibody Responses against SARS-CoV-2 in Plasma and Saliva from Vaccinated and Infected Individuals.medRxiv [Preprint]. 2021 Dec 7:2021.05.11.21256972. doi: 10.1101/2021.05.11.21256972. medRxiv. 2021. Update in: Front Immunol. 2021 Dec 20;12:759688. doi: 10.3389/fimmu.2021.759688. PMID: 34031663 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

