Thromboxane Mobilizes Insect Blood Cells to Infection Foci
- PMID: 34987515
- PMCID: PMC8720849
- DOI: 10.3389/fimmu.2021.791319
Thromboxane Mobilizes Insect Blood Cells to Infection Foci
Abstract
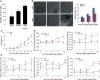
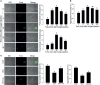
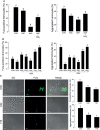
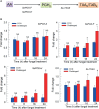
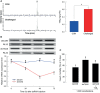
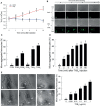
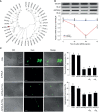
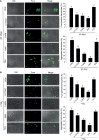
Innate immune responses are effective for insect survival to defend against entomopathogens including a fungal pathogen, Metarhizium rileyi, that infects a lepidopteran Spodoptera exigua. In particular, the fungal virulence was attenuated by cellular immune responses, in which the conidia were phagocytosed by hemocytes (insect blood cells) and hyphal growth was inhibited by hemocyte encapsulation. However, the chemokine signal to drive hemocytes to the infection foci was little understood. The hemocyte behaviors appeared to be guided by a Ca2+ signal stimulating cell aggregation to the infection foci. The induction of the Ca2+ signal was significantly inhibited by the cyclooxygenase (COX) inhibitor. Under the inhibitory condition, the addition of thromboxane A2 or B2 (TXA2 or TXB2) among COX products was the most effective to recover the Ca2+ signal and hemocyte aggregation. TXB2 alone induced a microaggregation behavior of hemocytes under in vitro conditions. Indeed, TXB2 titer was significantly increased in the plasma of the infected larvae. The elevated TXB2 level was further supported by the induction of phospholipase A2 (PLA2) activity in the hemocytes and subsequent up-regulation of COX-like peroxinectins (SePOX-F and SePOX-H) in response to the fungal infection. Finally, the expression of a thromboxane synthase (Se-TXAS) gene was highly expressed in the hemocytes. RNA interference (RNAi) of Se-TXAS expression inhibited the Ca2+ signal and hemocyte aggregation around fungal hyphae, which were rescued by the addition of TXB2. Without any ortholog to mammalian thromboxane receptors, a prostaglandin receptor was essential to mediate TXB2 signal to elevate the Ca2+ signal and mediate hemocyte aggregation behavior. Specific inhibitor assays suggest that the downstream signal after binding TXB2 to the receptor follows the Ca2+-induced Ca2+ release pathway from the endoplasmic reticulum of the hemocytes. These results suggest that hemocyte aggregation induced by the fungal infection is triggered by TXB2via a Ca2+ signal through a PG receptor.
Keywords: Spodoptera exigua; fungi; hemocyte; insect; thromboxane.
Copyright © 2021 Roy, Nam, Kim, Stanley and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The prostanoids, thromboxanes, mediate hemocytic immunity to bacterial infection in the lepidopteran Spodoptera exigua.Dev Comp Immunol. 2021 Jul;120:104069. doi: 10.1016/j.dci.2021.104069. Epub 2021 Mar 16. Dev Comp Immunol. 2021. PMID: 33737116
-
Nitric Oxide Mediates Insect Cellular Immunity via Phospholipase A2 Activation.J Innate Immun. 2018;10(1):70-81. doi: 10.1159/000481524. Epub 2017 Oct 17. J Innate Immun. 2018. PMID: 29035888 Free PMC article.
-
Toll signal pathway activating eicosanoid biosynthesis shares its conserved upstream recognition components in a lepidopteran Spodoptera exigua upon infection by Metarhizium rileyi, an entomopathogenic fungus.J Invertebr Pathol. 2022 Feb;188:107707. doi: 10.1016/j.jip.2021.107707. Epub 2021 Dec 21. J Invertebr Pathol. 2022. PMID: 34952100
-
Immunity in lepidopteran insects.Adv Exp Med Biol. 2010;708:181-204. doi: 10.1007/978-1-4419-8059-5_10. Adv Exp Med Biol. 2010. PMID: 21528699 Free PMC article. Review.
-
An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes.Virulence. 2013 Oct 1;4(7):597-603. doi: 10.4161/viru.25906. Epub 2013 Aug 6. Virulence. 2013. PMID: 23921374 Free PMC article. Review.
Cited by
-
Lipids as a key element of insect defense systems.Front Genet. 2023 Jun 9;14:1183659. doi: 10.3389/fgene.2023.1183659. eCollection 2023. Front Genet. 2023. PMID: 37359377 Free PMC article. Review.
-
Differences in honeybee queen pheromones revealed by LC-MS/MS: Reassessing the honest signal hypothesis.iScience. 2024 Sep 12;27(10):110906. doi: 10.1016/j.isci.2024.110906. eCollection 2024 Oct 18. iScience. 2024. PMID: 39391732 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

