Cepharanthine Blocks Presentation of Thyroid and Islet Peptides in a Novel Humanized Autoimmune Diabetes and Thyroiditis Mouse Model
- PMID: 34987519
- PMCID: PMC8721038
- DOI: 10.3389/fimmu.2021.796552
Cepharanthine Blocks Presentation of Thyroid and Islet Peptides in a Novel Humanized Autoimmune Diabetes and Thyroiditis Mouse Model
Abstract
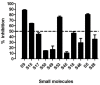
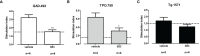
Autoimmune polyglandular syndrome type 3 variant (APS3v) refers to an autoimmune condition in which both type 1 diabetes (T1D) and autoimmune thyroiditis (AITD) develop in the same individual. HLA-DR3 confers the strongest susceptibility to APS3v. Previously we reported a unique amino acid signature pocket that predisposes to APS3v. We found that this pocket is flexible and can trigger APS3v by presenting both thyroid (Tg.1571, TPO.758) and islet (GAD.492) peptides to induce autoimmune response. We hypothesized that blocking the specific APS3v-HLA-DR3 pocket from presenting thyroid/islet antigens can block the autoimmune response in APS3v. To test this hypothesis we performed a virtual screen of small molecules blocking APS3v-HLA-DR3, and identified 11 small molecules hits that were predicted to block APS3v-HLA-DR3. Using the baculovirus-produced recombinant APS3v-HLA-DR3 protein we tested the 11 small molecules in an in vitro binding assay. We validated 4 small molecule hits, S9, S5, S53 and S15, that could block the APS3v-HLA-DR3 pocket in vitro. We then developed a novel humanized APS3v mouse model induced by co-immunizing a peptide mix of Tg.1571, TPO.758 and GAD.492. The immunized mice developed strong T-cell and antibody responses to the thyroid/islet peptides, as well as mouse thyroglobulin. In addition, the mice showed significantly lower free T4 levels compared to controls. Using the APS3v mouse model, we showed that one of the 4 small molecules, Cepharanthine (S53), blocked T-cell activation by thyroid/islet peptides ex vivo and in vivo. These findings suggested Cepharanthine may have a therapeutic potential in APS3v patients carrying the specific APS3v-HLA-DR3 pocket.
Keywords: autoimmune polyglandular syndrome; autoimmune thyroiditis; cepharanthine; immune therapy; type 1 diabetes.
Copyright © 2021 Li, Osman, Menconi, Faustino, Kim, Clarke, Hou and Tomer.
Conflict of interest statement
YT declares that he was previously (1/2015 – 6/2017) the principal investigator on a basic research project jointly funded by the Juvenile Diabetes Research Foundation and Pfizer. The current manuscript is not related to that research project. YT and CL declare that they submitted a patent application for Cepharanthine as a treatment for APS3v and T1D. YT, RO, and LF have additional patent applications that are not related to the content of this manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Flexible peptide recognition by HLA-DR triggers specific autoimmune T-cell responses in autoimmune thyroiditis and diabetes.J Autoimmun. 2017 Jan;76:1-9. doi: 10.1016/j.jaut.2016.09.007. Epub 2016 Sep 23. J Autoimmun. 2017. PMID: 27670087 Free PMC article.
-
Tg.2098 is a major human thyroglobulin T-cell epitope.J Autoimmun. 2010 Aug;35(1):45-51. doi: 10.1016/j.jaut.2010.01.004. Epub 2010 Mar 19. J Autoimmun. 2010. PMID: 20303712 Free PMC article.
-
Cepharanthine blocks TSH receptor peptide presentation by HLA-DR3: Therapeutic implications to Graves' disease.J Autoimmun. 2020 Mar;108:102402. doi: 10.1016/j.jaut.2020.102402. Epub 2020 Jan 21. J Autoimmun. 2020. PMID: 31980336 Free PMC article.
-
Application of HLA class II transgenic mice to study autoimmune regulation.Thyroid. 2007 Oct;17(10):995-1003. doi: 10.1089/thy.2007.0196. Thyroid. 2007. PMID: 17900224 Review.
-
Genetics of the autoimmune polyglandular syndrome type 3 variant.Thyroid. 2010 Jul;20(7):737-43. doi: 10.1089/thy.2010.1639. Thyroid. 2010. PMID: 20578896 Review.
Cited by
-
Effective Inhibition of Thyroid Antigen Presentation Using Retro-Inverso Peptides in Experimental Autoimmune Thyroiditis: A Pathway Toward Immune Therapies of Thyroid Autoimmunity.Thyroid. 2023 Apr;33(4):492-500. doi: 10.1089/thy.2022.0511. Epub 2023 Mar 20. Thyroid. 2023. PMID: 36762945 Free PMC article.
-
Genetics and epigenetics of autoimmune thyroid diseases: Translational implications.Best Pract Res Clin Endocrinol Metab. 2023 Mar;37(2):101661. doi: 10.1016/j.beem.2022.101661. Epub 2022 Apr 11. Best Pract Res Clin Endocrinol Metab. 2023. PMID: 35459628 Free PMC article. Review.
-
Research progress on pharmacological effects and mechanisms of cepharanthine and its derivatives.Naunyn Schmiedebergs Arch Pharmacol. 2023 Nov;396(11):2843-2860. doi: 10.1007/s00210-023-02537-y. Epub 2023 Jun 20. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37338575 Review.
-
Pharmacological Activity of Cepharanthine.Molecules. 2023 Jun 27;28(13):5019. doi: 10.3390/molecules28135019. Molecules. 2023. PMID: 37446681 Free PMC article. Review.
-
Significance of HLA in Graves' disease and Graves' orbitopathy in Asian and Caucasian populations - a systematic review.Front Immunol. 2023 Sep 28;14:1256922. doi: 10.3389/fimmu.2023.1256922. eCollection 2023. Front Immunol. 2023. PMID: 37841270 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

