Styrylchromones: Biological Activities and Structure-Activity Relationship
- PMID: 34987699
- PMCID: PMC8720608
- DOI: 10.1155/2021/2804521
Styrylchromones: Biological Activities and Structure-Activity Relationship
Retraction in
-
Retracted: Styrylchromones: Biological Activities and Structure-Activity Relationship.Oxid Med Cell Longev. 2024 Jan 9;2024:9810761. doi: 10.1155/2024/9810761. eCollection 2024. Oxid Med Cell Longev. 2024. PMID: 38234515 Free PMC article.
Abstract
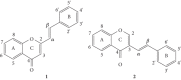
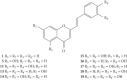
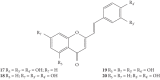
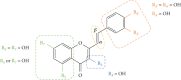
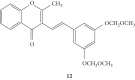
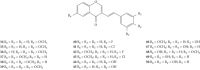
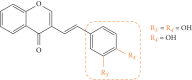
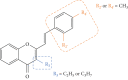
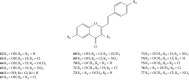
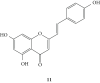
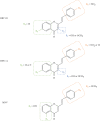
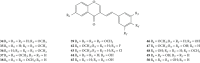
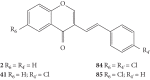
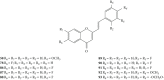
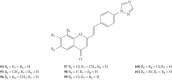
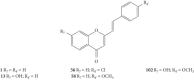
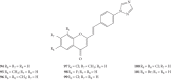
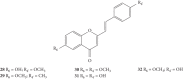
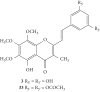
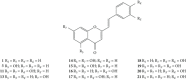
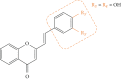
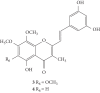
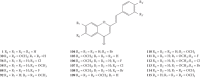
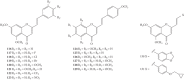
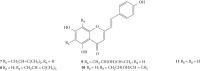
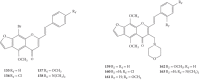
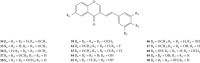
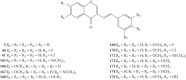
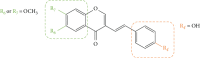
Styrylchromones (SC) are a group of oxygen-containing heterocyclic compounds, which are characterized by the attachment of a styryl group to the chromone core. SC can be found in nature or can be chemically synthesized in the laboratory. As their presence in nature is scarce, the synthetic origin is the most common. Two types of SC are known: 2-styrylchromones and 3-styrylchromones. However, 2-styrylchromones are the most common, being more commonly found in nature and which chemical synthesis is more commonly described. A wide variety of SC has been described in the literature, with different substituents in different positions, the majority of which are distributed on the A- and/or B-rings. Over the years, several biological activities have been attributed to SC. This work presents a comprehensive review of the biological activities attributed to SC and their structure-activity relationship, based on a published literature search, since 1989. The following biological activities are thoroughly revised and discussed in this review: antioxidant, antiallergic, antiviral, antibacterial, antifungal, anti-inflammatory, and antitumoral, affinity and selectivity for A3 adenosine receptors, neuroprotective, and α-glucosidase inhibition. In general, SC are composed by a promising scaffold with great potential for the development of new drugs.
Copyright © 2021 Mariana Lucas et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

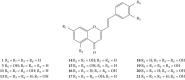
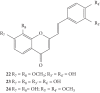
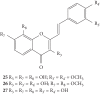
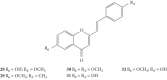















References
-
- Santos C. M. M., Silva A. M. S. An overview of 2-styrylchromones: natural occurrence, synthesis, reactivity and biological properties. European Journal of Organic Chemistry . 2017;2017(22):3115–3133. doi: 10.1002/ejoc.201700003. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

