Clinical relevance of abstruse transport phenomena in haemodialysis
- PMID: 34987788
- PMCID: PMC8711756
- DOI: 10.1093/ckj/sfab183
Clinical relevance of abstruse transport phenomena in haemodialysis
Abstract
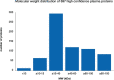
Haemodialysis (HD) utilizes the bidirectional properties of semipermeable membranes to remove uraemic toxins from blood while simultaneously replenishing electrolytes and buffers to correct metabolic acidosis. However, the nonspecific size-dependent transport across membranes also means that certain useful plasma constituents may be removed from the patient (together with uraemic toxins), or toxic compounds, e.g. endotoxin fragments, may accompany electrolytes and buffers of the dialysis fluids into blood and elicit severe biological reactions. We describe the mechanisms and implications of these undesirable transport processes that are inherent to all HD therapies and propose approaches to mitigate the effects of such transport. We focus particularly on two undesirable events that are considered to adversely affect HD therapy and possibly impact patient outcomes. Firstly, we describe how loss of albumin (and other essential substances) can occur while striving to eliminate larger uraemic toxins during HD and why hypoalbuminemia is a clinical condition to contend with. Secondly, we describe the origins and mode of transport of biologically active substances (from dialysis fluids with bacterial contamination) into the blood compartment and biological reactions they elicit. Endotoxin fragments activate various proinflammatory pathways to increase the underlying inflammation associated with chronic kidney disease. Both phenomena involve the physical as well as chemical properties of membranes that must be selected judiciously to balance the benefits with potential risks patients may encounter, in both the short and long term.
Keywords: albumin loss; endotoxin; haemodialysis membranes; hypoalbuminemia; transport.
© The Author(s) 2021. Published by Oxford University Press on behalf of ERA.
Figures




Similar articles
-
The membrane perspective of uraemic toxins: which ones should, or can, be removed?Clin Kidney J. 2021 Dec 27;14(Suppl 4):i17-i31. doi: 10.1093/ckj/sfab202. eCollection 2021 Dec. Clin Kidney J. 2021. PMID: 34987783 Free PMC article. Review.
-
The scientific principles and technological determinants of haemodialysis membranes.Clin Kidney J. 2021 Dec 27;14(Suppl 4):i5-i16. doi: 10.1093/ckj/sfab184. eCollection 2021 Dec. Clin Kidney J. 2021. PMID: 34987782 Free PMC article. Review.
-
Blood-incompatibility in haemodialysis: alleviating inflammation and effects of coagulation.Clin Kidney J. 2021 Dec 27;14(Suppl 4):i59-i71. doi: 10.1093/ckj/sfab185. eCollection 2021 Dec. Clin Kidney J. 2021. PMID: 34987786 Free PMC article. Review.
-
Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: a randomized clinical trial.Nephrol Dial Transplant. 2020 Feb 1;35(2):328-335. doi: 10.1093/ndt/gfz189. Nephrol Dial Transplant. 2020. PMID: 31578564 Clinical Trial.
-
Flummoxed by flux: the indeterminate principles of haemodialysis.Clin Kidney J. 2021 Dec 27;14(Suppl 4):i32-i44. doi: 10.1093/ckj/sfab182. eCollection 2021 Dec. Clin Kidney J. 2021. PMID: 34987784 Free PMC article. Review.
Cited by
-
Impact of Hydrophilic Modification of Synthetic Dialysis Membranes on Hemocompatibility and Performance.Membranes (Basel). 2022 Sep 26;12(10):932. doi: 10.3390/membranes12100932. Membranes (Basel). 2022. PMID: 36295691 Free PMC article. Review.
References
-
- Ronco C, Clark WR. Haemodialysis membranes. Nat Rev Nephrol 2018; 14: 394–410 - PubMed
-
- Mujais SK. Protein permeability in dialysis. Nephrol Dial Transplant 2000; 15: 10–14 - PubMed
-
- Lonnemann G. Should ultra-pure dialysate be mandatory? Nephrol Dial Transplant 2000; 15: 55–59 - PubMed
-
- Williams A. Hemodialysis and peritoneal dialysis. In: Godbole PP, Koyle MA, Wilcox DT (eds). Pediatric Urology: Surgical Complications and Management, 2nd edn. Hoboken, NJ: Wiley-Blackwell, 2015; 307–314
Publication types
LinkOut - more resources
Full Text Sources

