Early Experience With Tenecteplase at a Comprehensive Stroke Center
- PMID: 34987884
- PMCID: PMC8723952
- DOI: 10.1212/CPJ.0000000000001096
Early Experience With Tenecteplase at a Comprehensive Stroke Center
Abstract
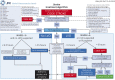
Purpose of review: Tenecteplase has been studied and recommended as an alternative thrombolytic agent in patients with acute stroke. A brief review of clinical trials and guidelines pertinent to our clinical decision algorithm is described. This is followed by operational steps that were made to create and implement a clinical pathway based on available evidence in which tenecteplase is used in select patients with stroke at our comprehensive stroke center.
Recent findings: A number of patients have been treated at our center with IV tenecteplase. A case is presented to illustrate the successful implementation of this new process.
Summary: Development of our protocol is discussed in detail to enable other centers to create their own clinical pathways for thrombolytic treatment of acute ischemic stroke using tenecteplase.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344-e418. - PubMed
-
- Logallo N, Kvistad CE, Thomassen L. Therapeutic potential of tenecteplase in the management of acute ischemic stroke. CNS Drugs. 2015;29:811-818. - PubMed
-
- Dunn CJ, Goa KL. Tenecteplase: a review of its pharmacology and therapeutic efficacy in patients with acute myocardial infarction. Am J Cardiovasc Drugs. 2001;1:51-66. - PubMed
-
- Van De Werf F, Adgey J, Ardissino D, et al. . Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716-722. - PubMed
-
- Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
