Europium Fluorescent Nanoparticles-Based Multiplex Lateral Flow Immunoassay for Simultaneous Detection of Three Antibiotic Families Residue
- PMID: 34988061
- PMCID: PMC8722402
- DOI: 10.3389/fchem.2021.793355
Europium Fluorescent Nanoparticles-Based Multiplex Lateral Flow Immunoassay for Simultaneous Detection of Three Antibiotic Families Residue
Abstract
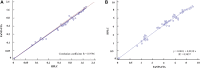
A fluorescent immunoassay based on europium nanoparticles (EuNPs-FIA) was developed for the simultaneous detection of antibiotic residues, solving the problems of single target detection and low sensitivity of traditional immunoassay methods. In the EuNPs-FIA, EuNPs were used as indictive probes by binding to anti-tetracyclines monoclonal antibodies (anti-TCs mAb), anti-sulphonamides monoclonal antibodies (anti-SAs mAb) and anti-fluoroquinolones monoclonal antibodies (anti-FQs mAb), respectively. Different artificial antigens were assigned to different regions of the nitrocellulose membrane as capture reagents. The EuNPs-FIA allowed for the simultaneous detection of three classes of antibiotics (tetracyclines, fluoroquinolones and sulphonamides) within 15 min. It enabled both the qualitative determination with the naked eye under UV light and the quantitative detection of target antibiotics by scanning the fluorescence intensity of the detection probes on the corresponding detection lines. For qualitative analysis, the cut-off values for tetracyclines (TCs), fluoroquinolones (FQs) and sulphonamides (SAs) were 3.2 ng/ml, 2.4 ng/ml and 4.0 ng/ml, respectively, which were much lower than the maximum residue limit in food. For quantitative analysis, these ranged from 0.06 to 6.85 ng/ml for TCs, 0.03-5.14 ng/ml for FQs, and 0.04-4.40 ng/ml for SAs. The linear correlation coefficients were higher than 0.97. The mean spiked recoveries ranged from 92.1 to 106.2% with relative standard deviations less than 8.75%. Among them, the three monoclonal antibodies could recognize four types of TCs, seven types of FQs and 13 types of SAs, respectively, and the detection range could cover 24 antibiotic residues with different structural formulations. The results of the detection of antibiotic residues in real samples using this method were highly correlated with those of high performance liquid chromatography (R 2 > 0.98). The accuracy and precision of the EuNPs-FIA also met the requirements for quantitative analysis. These results suggested that this multiplex immunoassay method was a promising method for rapid screening of three families of antibiotic residues.
Keywords: europium nanoparticle fluorescence immunochromatography; fluoroquinolone antibiotics; multi-residue detection; sulfonamide antibiotics; tetracycline antibiotics.
Copyright © 2021 Wang, Ma, Liu, Chen, Xu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Abafe O. A., Gatyeni P., Matika L. (2020). A Multi-Class Multi-Residue Method for the Analysis of Polyether Ionophores, Tetracyclines and Sulfonamides in Multi-Matrices of Animal and Aquaculture Fish Tissues by Ultra-high Performance Liquid Chromatography Tandem Mass Spectrometry. Food Additives & Contaminants: A 37, 438–450. 10.1080/19440049.2019.1705399 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

