Presynaptic Autophagy and the Connection With Neurotransmission
- PMID: 34988081
- PMCID: PMC8722708
- DOI: 10.3389/fcell.2021.790721
Presynaptic Autophagy and the Connection With Neurotransmission
Abstract
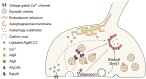
Autophagy is an evolutionary conserved catabolic pathway essential for the maintenance of cellular homeostasis. Defective proteins and organelles are engulfed by autophagosomal membranes which fuse with lysosomes for cargo degradation. In neurons, the orchestrated progression of autophagosome formation and maturation occurs in distinct subcellular compartments. For synapses, the distance from the soma and the oxidative stress generated during intense neuronal activity pose a challenge to maintain protein homeostasis. Autophagy constitutes a crucial mechanism for proper functioning of this unique and vulnerable cellular compartment. We are now beginning to understand how autophagy is regulated at pre-synaptic terminals and how this pathway, when imbalanced, impacts on synaptic function and -ultimately- neuronal survival. We review here the current state of the art of "synaptic autophagy", with an emphasis on the biogenesis of autophagosomes at the pre-synaptic compartment. We provide an overview of the existing knowledge on the signals inducing autophagy at synapses, highlight the interplay between autophagy and neurotransmission, and provide perspectives for future research.
Keywords: macroautophagy; neurotransmission; synapse; synaptic autophagy; vesicle cycling.
Copyright © 2021 Decet and Verstreken.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cases-Langhoff C., Voss B., Garner A. M., Appeltauer U., Takei K., Kindler S., et al. (1996). Piccolo, a Novel 420 kDa Protein Associated with the Presynaptic Cytomatrix. Eur. J. Cel Biol 69, 214–223. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

