AMP-activated protein kinase alpha1 promotes tumor development via FOXP3 elevation in tumor-infiltrating Treg cells
- PMID: 34988407
- PMCID: PMC8704466
- DOI: 10.1016/j.isci.2021.103570
AMP-activated protein kinase alpha1 promotes tumor development via FOXP3 elevation in tumor-infiltrating Treg cells
Abstract
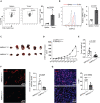
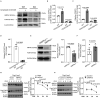
Overwhelming evidence indicates that infiltration of tumors by Treg cells with elevated levels of FOXP3 suppresses the host antitumor immune response. However, the molecular mechanisms that maintain high expression of FOXP3 in tumor-infiltrating Treg cells remain elusive. Here, we report that AMP-activated protein kinase alpha1 (AMPKα1) enables high FOXP3 expression in tumor-infiltrating Treg cells. Mice with Treg-specific AMPKα1 deletion showed delayed tumor progression and enhanced antitumor T cell immunity. Further experiments showed that AMPKα1 maintains the functional integrity of Treg cells and prevents interferon-γ production in tumor-infiltrating Treg cells. Mechanistically, AMPKα1 maintains the protein stability of FOXP3 in Treg cells by downregulating the expression of E3 ligase CHIP (STUB1). Our results suggest that AMPKα1 activation promotes tumor growth by maintaining FOXP3 stability in tumor-infiltrating Treg cells and that selective inhibition of AMPK in Treg cells might be an effective anti-tumor therapy.
Keywords: Cancer; Immunology; Molecular biology.
© 2021.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Bacchetta R., Barzaghi F., Roncarolo M.G. From IPEX syndrome to FOXP3 mutation: A lesson on immune dysregulation. Ann. N. Y Acad. Sci. 2018;1417:5–22. - PubMed
-
- Bennett C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., Ochs H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. - PubMed
-
- Borst J., Ahrends T., Babala N., Melief C.J.M., Kastenmuller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018;18:635–647. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

