Flux-Based Formulation Development-A Proof of Concept Study
- PMID: 34988721
- PMCID: PMC8816521
- DOI: 10.1208/s12248-021-00668-9
Flux-Based Formulation Development-A Proof of Concept Study
Abstract
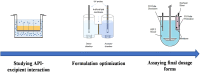
The work aimed to develop the Absorption Driven Drug Formulation (ADDF) concept, which is a new approach in formulation development to ensure that the drug product meets the expected absorption rate. The concept is built on the solubility-permeability interplay and the rate of supersaturation as the driving force of absorption. This paper presents the first case study using the ADDF concept where not only dissolution and solubility but also permeation of the drug is considered in every step of the formulation development. For that reason, parallel artificial membrane permeability assay (PAMPA) was used for excipient selection, small volume dissolution-permeation apparatus was used for testing amorphous solid dispersions (ASDs), and large volume dissolution-permeation tests were carried out to characterize the final dosage forms. The API-excipient interaction studies on PAMPA indicated differences when different fillers or surfactants were studied. These differences were then confirmed with small volume dissolution-permeation assays where the addition of Tween 80 to the ASDs decreased the flux dramatically. Also, the early indication of sorbitol's advantage over mannitol by PAMPA has been confirmed in the investigation of the final dosage forms by large-scale dissolution-permeation tests. This difference between the fillers was observed in vivo as well. The presented case study demonstrated that the ADDF concept opens a new perspective in generic formulation development using fast and cost-effective flux-based screening methods in order to meet the bioequivalence criteria. Graphical Abstract.
Keywords: absorption; dissolution-permeation; formulation additives; in vivo predictive; telmisartan.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- In Vitro Metabolismand Transporter Mediated Drug-Drug Interaction Studies Guidance for Industry, 2017. https://www.fda.gov/files/drugs/published/In-Vitro-Metabolism--and-Trans.... Accessed 04/07/2021.
-
- EMA. Guideline on the investigation of bioequivalence, London. J Physiol. 2010. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-in.... Accessed 03/04/2021.
-
- Borbás E, Balogh A, Bocz K, Müller J, Kiserdei É, Vigh T, Sinkó B, Marosi A, Halász A, Dohányos Z, Szente L, Balogh GT, Nagy ZK. In vitro dissolution-permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using μFluxTM. Int J Pharm. 2015;491:180–189. doi: 10.1016/j.ijpharm.2015.06.019. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

