Survival of Plants During Short-Term BOA-OH Exposure: ROS Related Gene Expression and Detoxification Reactions Are Accompanied With Fast Membrane Lipid Repair in Root Tips
- PMID: 34988771
- PMCID: PMC8881443
- DOI: 10.1007/s10886-021-01337-z
Survival of Plants During Short-Term BOA-OH Exposure: ROS Related Gene Expression and Detoxification Reactions Are Accompanied With Fast Membrane Lipid Repair in Root Tips
Abstract
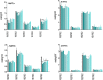
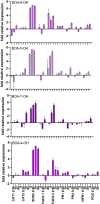
For the characterization of BOA-OH insensitive plants, we studied the time-dependent effects of the benzoxazolinone-4/5/6/7-OH isomers on maize roots. Exposure of Zea mays seedlings to 0.5 mM BOA-OH elicits root zone-specific reactions by the formation of dark rings and spots in the zone of lateral roots, high catalase activity on root hairs, and no visible defense reaction at the root tip. We studied BOA-6-OH- short-term effects on membrane lipids and fatty acids in maize root tips in comparison to the benzoxazinone-free species Abutilon theophrasti Medik. Decreased contents of phosphatidylinositol in A. theophrasti and phosphatidylcholine in maize were found after 10-30 min. In the youngest tissue, α-linoleic acid (18:2), decreased considerably in both species and recovered within one hr. Disturbances in membrane phospholipid contents were balanced in both species within 30-60 min. Triacylglycerols (TAGs) were also affected, but levels of maize diacylglycerols (DAGs) were almost unchanged, suggesting a release of fatty acids for membrane lipid regeneration from TAGs while resulting DAGs are buildings blocks for phospholipid reconstitution, concomitant with BOA-6-OH glucosylation. Expression of superoxide dismutase (SOD2) and of ER-bound oleoyl desaturase (FAD2-2) genes were contemporaneously up regulated in contrast to the catalase CAT1, while CAT3 was arguably involved at a later stage of the detoxification process. Immuno-responses were not elicited in short-terms, since the expression of NPR1, POX12 were barely affected, PR4 after 6 h with BOA-4/7-OH and PR1 after 24 h with BOA-5/6-OH. The rapid membrane recovery, reactive oxygen species, and allelochemical detoxification may be characteristic for BOA-OH insensitive plants.
Keywords: BOA-OH isomers; Detoxification; FAD2-2; Lipids; Membrane repair; ROS; SOD2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interest. The authors have no relevant financial or non-financial interests to disclose.
Figures









References
-
- Ali S, Ganai BA, Kamili AN, Bhat AA, Mir ZA, Bhat JA, Tyagi A, Islam ST, Mushtaq M, Yadav P, Rawat S, Grover A. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol Res. 2018;212–213:29–37. doi: 10.1016/j.micres.2018.04.008. - DOI - PubMed
-
- Baker CJ, Roberts DP, Mock NM, Whitaker BD, Deahl KL, Aver'yanov AA (2005) Apoplastic redox metabolism: Synergistic phenolic oxidation and a novel oxidative burst. Publications from USDA-ARS/UNL Faculty. 337. https://digitalcommons.unl.edu/usdaarsfacpub/337
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

