Neuronal regulation of glucagon secretion and gluconeogenesis
- PMID: 34989155
- PMCID: PMC9017634
- DOI: 10.1111/jdi.13745
Neuronal regulation of glucagon secretion and gluconeogenesis
Abstract
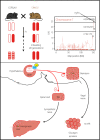
Hypoglycemia almost never develops in healthy individuals, because multiple hypoglycemia sensing systems, located in the periphery and in the central nervous system, trigger a coordinated counterregulatory hormonal response to restore normoglycemia. This involves not only the secretion of glucagon, but also of epinephrine, norepinephrine, cortisol and growth hormone. Increased hepatic glucose production is also stimulated by direct autonomous nervous connections to the liver that stimulate glycogenolysis and gluconeogenesis. This counterregulatory response, however, becomes deregulated in a significant fraction of diabetes patients that receive insulin therapy. This leads to the risk of developing hypoglycemic episodes, of increasing severity, which negatively impact the quality of life of the patients. How hypoglycemia is detected by the central nervous system is being actively investigated. Recent studies using novel molecular biological, optogenetic and chemogenetic techniques allow the characterization of glucose-sensing neurons, the mechanisms of hypoglycemia detection, the neuronal circuits in which they are integrated and the physiological responses they control. This review discusses recent studies aimed at identifying central hypoglycemia sensing neuronal circuits, how neurons are activated by hypoglycemia and how they restore normoglycemia.
Keywords: Glucagon; Hypoglycemia; Hypothalamus.
© 2022 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures


References
-
- Cryer PE. Mechanisms of hypoglycemia‐associated autonomic failure in diabetes. N Engl J Med 2013; 369: 362–372. - PubMed
-
- Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology 2007; 22: 241–251. - PubMed
-
- Anand BK, Chhina GS, Sharma KN, et al. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am J Physiol 1964; 207: 1146–1154. - PubMed
-
- Oomura Y, Yoshimatsu H. Neural network of glucose monitoring system. J Auton Nerv Syst 1984; 10: 359–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

