No Added Value of Duloxetine in Patients With Chronic Pain due to Hip or Knee Osteoarthritis: A Cluster-Randomized Trial
- PMID: 34989159
- PMCID: PMC9313808
- DOI: 10.1002/art.42040
No Added Value of Duloxetine in Patients With Chronic Pain due to Hip or Knee Osteoarthritis: A Cluster-Randomized Trial
Abstract
Objective: To assess the effectiveness of duloxetine in addition to usual care in patients with chronic osteoarthritis (OA) pain. The cost-effectiveness and whether the presence of symptoms of centralized pain alters the response to duloxetine were secondary objectives.
Methods: We conducted an open-label, cluster-randomized trial. Patients with chronic hip or knee OA pain who had an insufficient response to acetaminophen and nonsteroidal antiinflammatory drugs were included. Randomization took place at the general practice level, and patients received duloxetine (60 mg/day) in addition to usual care or usual care alone. The presence of centralized pain was defined as a modified PainDETECT Questionnaire score >12. The primary outcome measure was Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores (scale 0-20) at 3 months after the initiation of treatment. Our aim was to detect a difference between the groups of a clinically relevant effect of 1.9 points (effect size 0.4). We used a linear mixed model with repeated measurements to analyze the data.
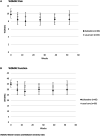
Results: In total, 133 patients were included, and 132 patients were randomized into treatment groups. A total of 66 patients (at 31 practices) were randomized to receive duloxetine in addition to usual care, and 66 patients (at 35 practices) were randomized to receive usual care alone. We found no differences in WOMAC pain scores between the groups at 3 months (adjusted difference -0.58 [95% confidence interval (95% CI) -1.80, 0.63]) or at 12 months (adjusted difference -0.26 [95% CI -1.86, 1.34]). In the subgroup of patients with centralized pain symptoms, we also found no effect of duloxetine compared to usual care alone (adjusted difference -0.32 [95% CI -2.32, 1.67]).
Conclusion: We found no effect of duloxetine added to usual care compared to usual care alone in patients with chronic knee or hip OA pain. Another trial including patients with centralized pain symptoms should be conducted to validate our results.
© 2022 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

Comment in
-
Duloxetine may have clinical value: comment on the article by van den Driest et al.Arthritis Rheumatol. 2022 Nov;74(11):1859-1860. doi: 10.1002/art.42293. Epub 2022 Oct 3. Arthritis Rheumatol. 2022. PMID: 35791999 No abstract available.
-
Duloxetine and osteoarthritis, a herd of elephants in the room: comment on the article by van den Driest et al.Arthritis Rheumatol. 2022 Nov;74(11):1859. doi: 10.1002/art.42294. Epub 2022 Oct 4. Arthritis Rheumatol. 2022. PMID: 35792040 No abstract available.
References
-
- Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 2014;28:5–15. - PubMed
-
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30. - PubMed
-
- Da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Juni P, et al. Effectiveness of non‐steroidal anti‐inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta‐analysis. Lancet 2017;390:e21–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

