Vitamin D3 and carbamazepine protect against Clostridioides difficile infection in mice by restoring macrophage lysosome acidification
- PMID: 34989311
- PMCID: PMC9466624
- DOI: 10.1080/15548627.2021.2016004
Vitamin D3 and carbamazepine protect against Clostridioides difficile infection in mice by restoring macrophage lysosome acidification
Abstract
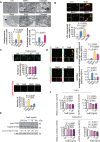
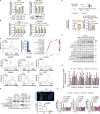
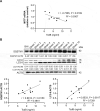
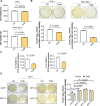
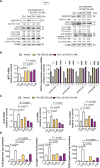
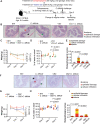
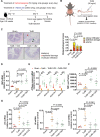
Clostridioides difficile infection (CDI) is a common cause of nosocomial diarrhea. TcdB is a major C. difficile exotoxin that activates macrophages to promote inflammation and epithelial damage. Lysosome impairment is a known trigger for inflammation. Herein, we hypothesize that TcdB could impair macrophage lysosomal function to mediate inflammation during CDI. Effects of TcdB on lysosomal function and the downstream pro-inflammatory SQSTM1/p62-NFKB (nuclear factor kappa B) signaling were assessed in cultured macrophages and in a murine CDI model. Protective effects of two lysosome activators (i.e., vitamin D3 and carbamazepine) were assessed. Results showed that TcdB inhibited CTNNB1/β-catenin activity to downregulate MITF (melanocyte inducing transcription factor) and its direct target genes encoding components of lysosomal membrane vacuolar-type ATPase, thereby suppressing lysosome acidification in macrophages. The resulting lysosomal dysfunction then impaired autophagic flux and activated SQSTM1-NFKB signaling to drive the expression of IL1B/IL-1β (interleukin 1 beta), IL8 and CXCL2 (chemokine (C-X-C motif) ligand 2). Restoring MITF function by enforced MITF expression or restoring lysosome acidification with 1α,25-dihydroxyvitamin D3 or carbamazepine suppressed pro-inflammatory cytokine expression in vitro. In mice, gavage with TcdB-hyperproducing C. difficile or injection of TcdB into ligated colon segments caused prominent MITF downregulation in macrophages. Vitamin D3 and carbamazepine lessened TcdB-induced lysosomal dysfunction, inflammation and histological damage. In conclusion, TcdB inhibits the CTNNB1-MITF axis to suppress lysosome acidification and activates the downstream SQSTM1-NFKB signaling in macrophages during CDI. Vitamin D3 and carbamazepine protect against CDI by restoring MITF expression and lysosomal function in mice.Abbreviations: ATP6V0B: ATPase H+ transporting V0 subunit b; ATP6V0C: ATPase H+ transporting V0 subunit c; ATP6V0E1: ATPase H+ transporting V0 subunit e1; ATP6V1H: ATPase H+ transporting V1 subunit H; CBZ: carbamazepine; CDI: C. difficile infection; CXCL: chemokine C-X-X motif ligand; IL: interleukin; LAMP1: lysosomal-associated membrane protein 1; LC3: microtubule-associated protein 1 light chain 3; LEF: lymphoid enhancer binding factor 1; MITF: melanocyte inducing transcription factor; NFKB: nuclear factor kappa B; PMA: phorbol 12-myristate 13-acetate; TcdA: Clostridial toxin A; TcdB: Clostridial toxin B; TFE3: transcription factor E3; TFEB: transcription factor EB.
Keywords: Autophagic flux; Clostridium difficile; MITF; macrophages; toxin B.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Wiegand PN, Nathwani D, Wilcox MH, et al. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect. 2012;81:1–14. - PubMed
-
- Goy SD, Olling A, Neumann D, et al. Human neutrophils are activated by a peptide fragment of Clostridium difficile toxin B presumably via formyl peptide receptor. Cell Microbiol. 2015;17:893–909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
