Tie2 Activation via VE-PTP Inhibition With Razuprotafib as an Adjunct to Latanoprost in Patients With Open Angle Glaucoma or Ocular Hypertension
- PMID: 34989803
- PMCID: PMC8742526
- DOI: 10.1167/tvst.11.1.7
Tie2 Activation via VE-PTP Inhibition With Razuprotafib as an Adjunct to Latanoprost in Patients With Open Angle Glaucoma or Ocular Hypertension
Abstract
Purpose: To evaluate the ocular hypotensive efficacy and safety of razuprotafib, a novel Tie2 activator, when used as an adjunct to latanoprost in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).
Methods: Subjects with OAG or OHT and an unmedicated IOP from ≥22 mm Hg to <36 mm Hg were randomized to one of three treatment arms: razuprotafib every day (QD) + latanoprost; razuprotafib twice daily (BID) + latanoprost; or latanoprost monotherapy. The primary endpoint was change in mean diurnal IOP from baseline at day 28.
Results: A total of 194 subjects were randomized, and 193 (99.5%) completed the study. Razuprotafib BID + latanoprost resulted in a significantly larger reduction in diurnal IOP than latanoprost alone (7.95 ± 0.26 mmHg vs. 7.04 ± 0.26 mm Hg, P < 0.05). A smaller improvement was observed after 14 days of treatment (7.62 ± 0.26 mm Hg vs. 7.03 ± 0.26 mm Hg, P = 0.11). Razuprotafib QD dosing did not demonstrate additional IOP lowering compared to latanoprost alone. Conjunctival hyperemia on Day 28 increased by 1.1 units on the four-point Efron scale two hours post dose from a baseline value of 0.6 units, and decreased thereafter.
Conclusions: Topical ocular razuprotafib as an adjunct to latanoprost therapy was well tolerated and significantly reduced IOP in patients with OAG/OHT.
Translational relevance: These data support the IOP lowering efficacy of targeting Tie2 activation in Schlemm's canal in the relevant patient population.
Conflict of interest statement
Disclosure:
Figures

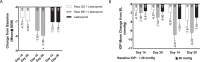


Similar articles
-
Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure.Ophthalmology. 2015 Feb;122(2):302-7. doi: 10.1016/j.ophtha.2014.08.022. Epub 2014 Sep 27. Ophthalmology. 2015. PMID: 25270273 Clinical Trial.
-
Comparison of brimonidine with latanoprost in the adjunctive treatment of glaucoma. ALPHAGAN/XALATAN Study Group.Clin Ther. 2000 Apr;22(4):388-99. doi: 10.1016/s0149-2918(00)89008-6. Clin Ther. 2000. PMID: 10823361 Clinical Trial.
-
Comparison of latanoprost with fixed-combination dorzolamide and timolol in adult patients with elevated intraocular pressure: an eight-week, randomized, open-label, parallel-group, multicenter study in Latin America.Clin Ther. 2004 May;26(5):755-68. doi: 10.1016/s0149-2918(04)90075-6. Clin Ther. 2004. PMID: 15220019 Clinical Trial.
-
Latanoprost : an update of its use in glaucoma and ocular hypertension.Drugs Aging. 2003;20(8):597-630. doi: 10.2165/00002512-200320080-00005. Drugs Aging. 2003. PMID: 12795627 Review.
-
Human experience and efficacy of omidenepag isopropyl (Eybelis®; Omlonti®): Discovery to approval of the novel non-prostaglandin EP2-receptor-selective agonist ocular hypotensive drug.Curr Opin Pharmacol. 2024 Feb;74:102426. doi: 10.1016/j.coph.2023.102426. Epub 2024 Jan 1. Curr Opin Pharmacol. 2024. PMID: 38168596 Review.
Cited by
-
Lipid Nanoparticle Delivery System for Normalization of Tumor Microenvironment and Tumor Vascular Structure.Biomater Res. 2025 Feb 11;29:0144. doi: 10.34133/bmr.0144. eCollection 2025. Biomater Res. 2025. PMID: 39935791 Free PMC article.
-
Shikonin as a therapeutic agent in renal cell carcinoma: insights from TEK-related causal association with glaucoma.Front Pharmacol. 2025 Jul 30;16:1580704. doi: 10.3389/fphar.2025.1580704. eCollection 2025. Front Pharmacol. 2025. PMID: 40808675 Free PMC article.
-
Penile cavernous sinusoids are Prox1-positive hybrid vessels.Vasc Biol. 2024 Jan 11;6(1):e230014. doi: 10.1530/VB-23-0014. Print 2024 Jan 1. Vasc Biol. 2024. PMID: 38051669 Free PMC article.
-
TIE1 and TEK signalling, intraocular pressure, and primary open-angle glaucoma: a Mendelian randomization study.J Transl Med. 2023 Nov 24;21(1):847. doi: 10.1186/s12967-023-04737-9. J Transl Med. 2023. PMID: 37996923 Free PMC article.
-
Identification of immune-related biomarkers for glaucoma using gene expression profiling.Front Genet. 2024 Apr 17;15:1366453. doi: 10.3389/fgene.2024.1366453. eCollection 2024. Front Genet. 2024. PMID: 38694874 Free PMC article.
References
-
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. - PubMed
-
- Vajaranant TS, Wu S, Torres M, Varma RA. 40-year forecast of the demographic shift in primary open-angle glaucoma in the United States. Invest Ophthalmol Vis Sci. 2012; 53: 2464–2466. - PubMed
-
- Gordon MO, Beiser JA, Brandt JD, et al. .. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 714–720; discussion 829-730. - PubMed
-
- Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120: 1268–1279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

