The sociodemographic and clinical profile of patients with major depressive disorder receiving SSRIs as first-line antidepressant treatment in European countries
- PMID: 34989830
- PMCID: PMC9095529
- DOI: 10.1007/s00406-021-01368-3
The sociodemographic and clinical profile of patients with major depressive disorder receiving SSRIs as first-line antidepressant treatment in European countries
Abstract
Introduction: Due to favorable antidepressant (AD) efficacy and tolerability, selective-serotonin reuptake inhibitors (SSRIs) are consistently recommended as substances of first choice for the treatment of major depressive disorder (MDD) in international guidelines. However, little is known about the real-world clinical correlates of patients primarily prescribed SSRIs in contrast to those receiving alternative first-line ADs.
Methods: These secondary analyses are based on a naturalistic, multinational cross-sectional study conducted by the European Group for the Study of Resistant Depression at ten research sites. We compared the socio-demographic and clinical characteristics of 1410 patients with primary MDD, who were either prescribed SSRIs or alternative substances as first-line AD treatment, using chi-squared tests, analyses of covariance, and logistic regression analyses.
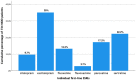
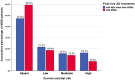
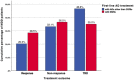
Results: SSRIs were prescribed in 52.1% of MDD patients who showed lower odds for unemployment, current severity of depressive symptoms, melancholic features, suicidality, as well as current inpatient treatment compared to patients receiving alternative first-line ADs. Furthermore, patients prescribed SSRIs less likely received add-on therapies including AD combination and augmentation with antipsychotics, and exhibited a trend towards higher response rates.
Conclusion: A more favorable socio-demographic and clinical profile associated with SSRIs in contrast to alternative first-line ADs may have guided European psychiatrists' treatment choice for SSRIs, rather than any relevant pharmacological differences in mechanisms of action of the investigated ADs. Our results must be cautiously interpreted in light of predictable biases resulting from the open treatment selection, the possible allocation of less severely ill patients to SSRIs as well as the cross-sectional study design that does not allow to ascertain any causal conclusions.
Keywords: Antidepressant treatment; Antidepressants; Major depressive disorder; Selective-serotonin reuptake inhibitors.
© 2022. The Author(s).
Conflict of interest statement
Dr. Bartova has received travel grants and consultant/speaker honoraria from AOP Orphan, Medizin Medien Austria, Vertretungsnetz, Schwabe Austria, Janssen and Angelini. Dr. Fugger has received consultant/speaker honoraria from Janssen. Dr. Dold has received travel grants and consultant/speaker honoraria from Janssen-Cilag. Dr. Zohar has received grant/research support from Lundbeck, Servier, and Pfizer; he has served as a consultant or on the advisory boards for Servier, Pfizer, Solvay, and Actelion; and he has served on speakers’ bureaus for Lundbeck, GlaxoSmithKline, Jazz, and Solvay. Dr. Mendlewicz is a member of the board of the Lundbeck International Neuroscience Foundation and of the advisory board of Servier. Dr. Souery has received grant/research support from GlaxoSmithKline and Lundbeck; and he has served as a consultant or on advisory boards for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, and Lundbeck. Dr. Montgomery has served as a consultant or on advisory boards for AstraZeneca, Bionevia, Bristol-Myers Squibb, Forest, GlaxoSmithKline, Grunenthal, Intellect Pharma, Johnson & Johnson, Lilly, Lundbeck, Merck, Merz, M's Science, Neurim, Otsuka, Pierre Fabre, Pfizer, Pharmaneuroboost, Richter, Roche, Sanofi, Sepracor, Servier, Shire, Synosis, Takeda, Theracos, Targacept, Transcept, UBC, Xytis, and Wyeth. Dr. Fabbri has been supported by Fondazione Umberto Veronesi (
Figures
Similar articles
-
Real-world characteristics of European patients receiving SNRIs as first-line treatment for major depressive disorder.J Affect Disord. 2023 Jul 1;332:105-114. doi: 10.1016/j.jad.2023.03.068. Epub 2023 Mar 22. J Affect Disord. 2023. PMID: 36958488
-
Melancholic features in major depression - a European multicenter study.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Aug 30;110:110285. doi: 10.1016/j.pnpbp.2021.110285. Epub 2021 Feb 18. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 33609603
-
Evidence on sociodemographic and clinical correlates of antidepressant combination or augmentation with second-generation antipsychotics in major depressive disorder.Prog Neuropsychopharmacol Biol Psychiatry. 2022 Mar 2;114:110480. doi: 10.1016/j.pnpbp.2021.110480. Epub 2021 Nov 23. Prog Neuropsychopharmacol Biol Psychiatry. 2022. PMID: 34826558
-
Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents.Biol Psychiatry. 2007 Dec 1;62(11):1217-27. doi: 10.1016/j.biopsych.2007.03.027. Epub 2007 Jun 22. Biol Psychiatry. 2007. PMID: 17588546 Review.
-
Vortioxetine: a New Treatment for Major Depressive Disorder.Expert Opin Pharmacother. 2016;17(3):421-31. doi: 10.1517/14656566.2016.1133588. Expert Opin Pharmacother. 2016. PMID: 26679430 Review.
Cited by
-
Mood Alterations in the Prodromal Phase of Sporadic Creutzfeldt-Jakob Disease.JAMA Neurol. 2025 Feb 1;82(2):185-192. doi: 10.1001/jamaneurol.2024.4447. JAMA Neurol. 2025. PMID: 39786417
-
Glial-Restricted Precursors stimulate endogenous cytogenesis and effectively recover emotional deficits in a model of cytogenesis ablation.Res Sq [Preprint]. 2023 Mar 31:rs.3.rs-2747462. doi: 10.21203/rs.3.rs-2747462/v1. Res Sq. 2023. Update in: Mol Psychiatry. 2024 Jul;29(7):2185-2198. doi: 10.1038/s41380-024-02490-z. PMID: 37034743 Free PMC article. Updated. Preprint.
-
Is Pilates effective in improving depressive disorders? A comprehensive overview.Int Clin Psychopharmacol. 2025 Mar 1;40(2):53-61. doi: 10.1097/YIC.0000000000000541. Epub 2024 Feb 29. Int Clin Psychopharmacol. 2025. PMID: 38277272 Free PMC article. Review.
-
Glial-restricted precursors stimulate endogenous cytogenesis and effectively recover emotional deficits in a model of cytogenesis ablation.Mol Psychiatry. 2024 Jul;29(7):2185-2198. doi: 10.1038/s41380-024-02490-z. Epub 2024 Mar 7. Mol Psychiatry. 2024. PMID: 38454085 Free PMC article.
-
Acute toxicity and genotoxicity studies on new melatonergic antidepressant GW117.Heliyon. 2023 Feb 24;9(3):e14026. doi: 10.1016/j.heliyon.2023.e14026. eCollection 2023 Mar. Heliyon. 2023. PMID: 36915542 Free PMC article.
References
-
- Ball S, Classi P, Dennehy EB. What happens next? A claims database study of second-line pharmacotherapy in patients with major depressive disorder (mdd) who initiate selective serotonin reuptake inhibitor (SSRI) treatment. Ann Gen Psychiatry. 2014;13:8. doi: 10.1186/1744-859X-13-8. - DOI - PMC - PubMed
-
- Bartova L, Dold M, Kautzky A, Fabbri C, Spies M, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, Schosser A, Kasper S. Results of the european group for the study of resistant depression (gsrd)—basis for further research and clinical practice. World J Biol Psychiatry. 2019;20:427–448. doi: 10.1080/15622975.2019.1635270. - DOI - PubMed
-
- Bartova L, Fugger G, Dold M, Swoboda MMM, Zohar J, Mendlewicz J, Souery D, Montgomery S, Fabbri C, Serretti A, Kasper S. Combining psychopharmacotherapy and psychotherapy is not associated with better treatment outcome in major depressive disorder—evidence from the european group for the study of resistant depression. J Psychiatr Res. 2021;141:167–175. doi: 10.1016/j.jpsychires.2021.06.028. - DOI - PubMed
-
- Bauer M, Severus E, Möller HJ, Young AH, WFSBP Task Force on Unipolar Depressive Disorders (2017) Pharmacological treatment of unipolar depressive disorders: summary of WFSBP guidelines. Int J Psychiatry Clin Pract 21(3):166–176 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

