Shrew's venom quickly causes circulation disorder, analgesia and hypokinesia
- PMID: 34989866
- PMCID: PMC11071750
- DOI: 10.1007/s00018-021-04116-x
Shrew's venom quickly causes circulation disorder, analgesia and hypokinesia
Abstract
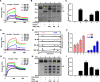
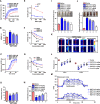
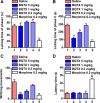
Multiple representatives of eulipotyphlan mammals such as shrews have oral venom systems. Venom facilitates shrews to hunt and/or hoard preys. However, little is known about their venom composition, and especially the mechanism to hoard prey in comatose states for meeting their extremely high metabolic rates. A toxin (BQTX) was identified from venomous submaxillary glands of the shrew Blarinella quadraticauda. BQTX is specifically distributed and highly concentrated (~ 1% total protein) in the organs. BQTX shares structural and functional similarities to toxins from snakes, wasps and snails, suggesting an evolutional relevancy of venoms from mammalians and non-mammalians. By potentiating thrombin and factor-XIIa and inhibiting plasmin, BQTX induces acute hypertension, blood coagulation and hypokinesia. It also shows strong analgesic function by inhibiting elastase. Notably, the toxin keeps high plasma stability with a 16-h half-life in-vivo, which likely extends intoxication to paralyze or immobilize prey hoarded fresh for later consumption and maximize foraging profit.
Keywords: Blarinella quadraticauda; Thrombin. FXIIa; Toxin; Venom.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
A Comprehensive Multi-Omic Approach Reveals a Relatively Simple Venom in a Diet Generalist, the Northern Short-Tailed Shrew, Blarina brevicauda.Genome Biol Evol. 2020 Jul 1;12(7):1148-1166. doi: 10.1093/gbe/evaa115. Genome Biol Evol. 2020. PMID: 32520994 Free PMC article.
-
Blarina toxin, a mammalian lethal venom from the short-tailed shrew Blarina brevicauda: Isolation and characterization.Proc Natl Acad Sci U S A. 2004 May 18;101(20):7542-7. doi: 10.1073/pnas.0402517101. Epub 2004 May 10. Proc Natl Acad Sci U S A. 2004. PMID: 15136743 Free PMC article.
-
Venom Use in Eulipotyphlans: An Evolutionary and Ecological Approach.Toxins (Basel). 2021 Mar 22;13(3):231. doi: 10.3390/toxins13030231. Toxins (Basel). 2021. PMID: 33810196 Free PMC article. Review.
-
A Polychaete's powerful punch: venom gland transcriptomics of Glycera reveals a complex cocktail of toxin homologs.Genome Biol Evol. 2014 Sep 5;6(9):2406-23. doi: 10.1093/gbe/evu190. Genome Biol Evol. 2014. PMID: 25193302 Free PMC article.
-
The evolutionary dynamics of venom toxins made by insects and other animals.Biochem Soc Trans. 2020 Aug 28;48(4):1353-1365. doi: 10.1042/BST20190820. Biochem Soc Trans. 2020. PMID: 32756910 Review.
Cited by
-
The Fast and the Furriest: Investigating the Rate of Selection on Mammalian Toxins.Toxins (Basel). 2022 Dec 1;14(12):842. doi: 10.3390/toxins14120842. Toxins (Basel). 2022. PMID: 36548740 Free PMC article.
-
Slowly Making Sense: A Review of the Two-Step Venom System within Slow (Nycticebus spp.) and Pygmy Lorises (Xanthonycticebus spp.).Toxins (Basel). 2023 Aug 22;15(9):514. doi: 10.3390/toxins15090514. Toxins (Basel). 2023. PMID: 37755940 Free PMC article. Review.
-
Isolation and Characterization of Poeciguamerin, a Peptide with Dual Analgesic and Anti-Thrombotic Activity from the Poecilobdella manillensis Leech.Int J Mol Sci. 2023 Jul 4;24(13):11097. doi: 10.3390/ijms241311097. Int J Mol Sci. 2023. PMID: 37446275 Free PMC article.
-
Viruses Identified in Shrews (Soricidae) and Their Biomedical Significance.Viruses. 2024 Sep 10;16(9):1441. doi: 10.3390/v16091441. Viruses. 2024. PMID: 39339918 Free PMC article. Review.
-
Proteins from shrews' venom glands play a role in gland functioning and venom production.Zoological Lett. 2024 Jul 15;10(1):12. doi: 10.1186/s40851-024-00236-x. Zoological Lett. 2024. PMID: 39010181 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 31930015/Innovative Research Group Project of the National Natural Science Foundation of China
- 32100907/Innovative Research Group Project of the National Natural Science Foundation of China
- 81770464/Innovative Research Group Project of the National Natural Science Foundation of China
- 32070443/Innovative Research Group Project of the National Natural Science Foundation of China
- XDB31000000/Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences and Peking Union Medical College
- KFJ-STS-SCYD-304/Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences and Peking Union Medical College
- 2019FA006/Applied Basic Research Foundation of Yunnan Province
- 2019FB127/Cultivating Plan Program for the Leader in Science and Technology of Yunnan Province
- 202003AD150008/Cultivating Plan Program for the Leader in Science and Technology of Yunnan Province
- 2019ZF003/Cultivating Plan Program for the Leader in Science and Technology of Yunnan Province
- 2018YFA0801403/Ministry of Science and Technology of the People's Republic of China
LinkOut - more resources
Full Text Sources

