Central Nervous System Control of Glucose Homeostasis: A Therapeutic Target for Type 2 Diabetes?
- PMID: 34990204
- PMCID: PMC8900291
- DOI: 10.1146/annurev-pharmtox-052220-010446
Central Nervous System Control of Glucose Homeostasis: A Therapeutic Target for Type 2 Diabetes?
Abstract
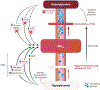
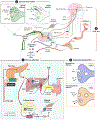
Historically, pancreatic islet beta cells have been viewed as principal regulators of glycemia, with type 2 diabetes (T2D) resulting when insulin secretion fails to compensate for peripheral tissue insulin resistance. However, glycemia is also regulated by insulin-independent mechanisms that are dysregulated in T2D. Based on evidence supporting its role both in adaptive coupling of insulin secretion to changes in insulin sensitivity and in the regulation of insulin-independent glucose disposal, the central nervous system (CNS) has emerged as a fundamental player in glucose homeostasis. Here, we review and expand upon an integrative model wherein the CNS, together with the islet, establishes and maintains the defended level of glycemia. We discuss the implications of this model for understanding both normal glucose homeostasis and T2D pathogenesis and highlight centrally targeted therapeutic approaches with the potential to restore normoglycemia to patients with T2D.
Keywords: autonomic nervous system; central nervous system; diabetes; glucose effectiveness; glucose homeostasis; insulin-independent glucose disposal; therapeutics.
Figures


References
-
- Turner RC, Holman RR, Matthews D, Hockaday TDR, Peto J. 1979. Insulin deficiency and insulin resistance interaction in diabetes: estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolis 28(11):1086–96 - PubMed
-
- Edelman SV, Polonsky WH. 2017. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care 40(11):1425–32 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

