Human CD206+ macrophages associate with diabetes and adipose tissue lymphoid clusters
- PMID: 34990410
- PMCID: PMC8855803
- DOI: 10.1172/jci.insight.146563
Human CD206+ macrophages associate with diabetes and adipose tissue lymphoid clusters
Abstract
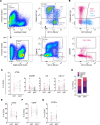
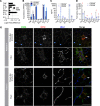
Increased adipose tissue macrophages (ATMs) correlate with metabolic dysfunction in humans and are causal in development of insulin resistance in mice. Recent bulk and single-cell transcriptomics studies reveal a wide spectrum of gene expression signatures possible for macrophages that depends on context, but the signatures of human ATM subtypes are not well defined in obesity and diabetes. We profiled 3 prominent ATM subtypes from human adipose tissue in obesity and determined their relationship to type 2 diabetes. Visceral adipose tissue (VAT) and s.c. adipose tissue (SAT) samples were collected from diabetic and nondiabetic obese participants to evaluate cellular content and gene expression. VAT CD206+CD11c- ATMs were increased in diabetic participants, were scavenger receptor-rich with low intracellular lipids, secreted proinflammatory cytokines, and diverged significantly from 2 CD11c+ ATM subtypes, which were lipid-laden, were lipid antigen presenting, and overlapped with monocyte signatures. Furthermore, diabetic VAT was enriched for CD206+CD11c- ATM and inflammatory signatures, scavenger receptors, and MHC II antigen presentation genes. VAT immunostaining found CD206+CD11c- ATMs concentrated in vascularized lymphoid clusters adjacent to CD206-CD11c+ ATMs, while CD206+CD11c+ were distributed between adipocytes. Our results show ATM subtype-specific profiles that uniquely contribute to the phenotypic variation in obesity.
Keywords: Adipose tissue; Diabetes; Immunology; Macrophages; Metabolism.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

