Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response amongst individuals in clinical risk groups
- PMID: 34990709
- PMCID: PMC8720678
- DOI: 10.1016/j.jinf.2021.12.044
Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response amongst individuals in clinical risk groups
Abstract
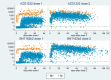
Background COVID-19 vaccines approved in the UK are highly effective in general population cohorts, however, data on effectiveness amongst individuals with clinical conditions that place them at increased risk of severe disease are limited. Methods We used GP electronic health record data, sentinel virology swabbing and antibody testing within a cohort of 712 general practices across England to estimate vaccine antibody response and vaccine effectiveness against medically attended COVID-19 amongst individuals in clinical risk groups using cohort and test-negative case control designs. Findings There was no reduction in S-antibody positivity in most clinical risk groups, however reduced S-antibody positivity and response was significant in the immunosuppressed group. Reduced vaccine effectiveness against clinical disease was also noted in the immunosuppressed group; after a second dose, effectiveness was moderate (Pfizer: 59.6%, 95%CI 18.0-80.1%; AstraZeneca 60.0%, 95%CI -63.6-90.2%). Interpretation In most clinical risk groups, immune response to primary vaccination was maintained and high levels of vaccine effectiveness were seen. Reduced antibody response and vaccine effectiveness were seen after 1 dose of vaccine amongst a broad immunosuppressed group, and second dose vaccine effectiveness was moderate. These findings support maximising coverage in immunosuppressed individuals and the policy of prioritisation of this group for third doses.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest Simon de Lusignan is the Director of the Oxford-RCGP RSC and has received funding through his University for studies from Astra-Zeneca, Eli Lilly, Sanofi, GSK, MSD. Seqirus and Takeda; and been member of advisory boards for Astra-Zeneca, Seqirus and Sanofi. Ezra Linley reports that the UKHSA Vaccine Evaluation Unit performs contract research on behalf of GSK, Sanofi and Pfizer which is outside the submitted work.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical