Association Between Menstrual Cycle Length and Coronavirus Disease 2019 (COVID-19) Vaccination: A U.S. Cohort
- PMID: 34991109
- PMCID: PMC8936155
- DOI: 10.1097/AOG.0000000000004695
Association Between Menstrual Cycle Length and Coronavirus Disease 2019 (COVID-19) Vaccination: A U.S. Cohort
Abstract
Objective: To assess whether coronavirus disease 2019 (COVID-19) vaccination is associated with changes in cycle or menses length in those receiving vaccination as compared with an unvaccinated cohort.
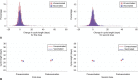
Methods: We analyzed prospectively tracked menstrual cycle data using the application "Natural Cycles." We included U.S. residents aged 18-45 years with normal cycle lengths (24-38 days) for three consecutive cycles before the first vaccine dose followed by vaccine-dose cycles (cycles 4-6) or, if unvaccinated, six cycles over a similar time period. We calculated the mean within-individual change in cycle and menses length (three prevaccine cycles vs first- and second-dose cycles in the vaccinated cohort, and the first three cycles vs cycles four and five in the unvaccinated cohort). We used mixed-effects models to estimate the adjusted difference in change in cycle and menses length between the vaccinated and unvaccinated cohorts.
Results: We included 3,959 individuals (vaccinated 2,403; unvaccinated 1,556). Most of the vaccinated cohort received the Pfizer-BioNTech vaccine (55%) (Moderna 35%, Johnson & Johnson/Janssen 7%). Overall, COVID-19 vaccine was associated with a less than 1-day change in cycle length for both vaccine-dose cycles compared with prevaccine cycles (first dose 0.71 day-increase, 98.75% CI 0.47-0.94; second dose 0.91, 98.75% CI 0.63-1.19); unvaccinated individuals saw no significant change compared with three baseline cycles (cycle four 0.07, 98.75% CI -0.22 to 0.35; cycle five 0.12, 98.75% CI -0.15 to 0.39). In adjusted models, the difference in change in cycle length between the vaccinated and unvaccinated cohorts was less than 1 day for both doses (difference in change: first dose 0.64 days, 98.75% CI 0.27-1.01; second dose 0.79 days, 98.75% CI 0.40-1.18). Change in menses length was not associated with vaccination.
Conclusion: Coronavirus disease 2019 (COVID-19) vaccination is associated with a small change in cycle length but not menses length.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Financial Disclosure Alison Edelman reports honoraria and travel reimbursement from ACOG, WHO, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. Alison Edelman receives royalties from UpToDate, Inc. Oregon Health & Science University (OHSU) receives research funding from OHSU Foundation, Merck, HRA Pharma, and NIH for which Alison Edelman is the principal investigator. Blair G. Darney reports honoraria and travel reimbursement from ACOG and SFP for board, committee, and mentorship activities. OHSU receives research funding from Merck/Organon and OPA/DHHS for which Blair G. Darney is the principal investigator. OHSU receives research funding from OHSU foundation, the Bill & Melinda Gates Foundation, ABOG, ASRM and the NIH for which Leo Han is the principal investigator. Eleonora Benhar, Carlotta Favaro, and Jack T. Pearson are employees of Natural Cycles. Kristen A. Matteson reports honoraria and travel reimbursement from ABOG and travel reimbursement from ACOG. Women & Infants Hospital received funding from Myovant for consulting work done by Kristen A. Matteson on outcomes measures for heavy menstrual bleeding. Emily R. Boniface did not report any potential conflicts of interest.
Figures
Comment in
-
Coronavirus Disease 2019 (COVID-19) Vaccination in Obstetrics and Gynecology: Addressing Concerns While Paving a Way Forward.Obstet Gynecol. 2022 Apr 1;139(4):479-480. doi: 10.1097/AOG.0000000000004715. Epub 2022 Jan 28. Obstet Gynecol. 2022. PMID: 35104258 No abstract available.
-
Association Between Menstrual Cycle Length and Coronavirus Disease 2019 (COVID-19) Vaccination: A U.S. Cohort.Obstet Gynecol. 2022 May 1;139(5):940-941. doi: 10.1097/AOG.0000000000004781. Obstet Gynecol. 2022. PMID: 35576361 No abstract available.
-
Does COVID-19 Vaccination Disturb Menstrual Cycling?Am J Epidemiol. 2023 Jun 2;192(6):849-850. doi: 10.1093/aje/kwad039. Am J Epidemiol. 2023. PMID: 36799647
-
Response to "Vaccination and the Menstrual Cycle".Am J Epidemiol. 2023 Jun 2;192(6):851-852. doi: 10.1093/aje/kwad049. Am J Epidemiol. 2023. PMID: 36883904 Free PMC article. No abstract available.
References
-
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The Advisory Committee on Immunization Practices' interim recommendation for use of Moderna COVID-19 vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1653–6. doi: 10.15585/mmwr.mm695152e1 - DOI - PMC - PubMed
-
- NOT-HD-21-035: notice of special interest (NOSI) to encourage administrative supplement applications to investigate COVID-19 vaccination and menstruation (admin supp clinical trial optional). Accessed June 12, 2021. https://grants.nih.gov/grants/guide/notice-files/NOT-HD-21-035.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

