A survey of patients with laryngotracheal stenosis on future clinical trial design
- PMID: 34991364
- PMCID: PMC9382824
- DOI: 10.1177/17407745211065744
A survey of patients with laryngotracheal stenosis on future clinical trial design
Abstract
Background/aims: Laryngotracheal stenosis is a rare but devastating proximal airway fibrosis that restricts a patient's ability to breathe. Treatment is primarily surgical and to date, there has never been a multi-institutional, randomized, prospective, and interventional clinical trial for a medical therapy to treat laryngotracheal stenosis. Therefore, we aimed to obtain patient feedback to guide successful trial design, recruitment, retention, and for identifying potential barriers to study participation.
Methods: Over 1000 members of an international laryngotracheal stenosis online support community (the Living with Idiopathic Subglottic Stenosis Facebook group) were sent two questionnaires for a proposed interventional double-blinded, randomized, placebo-controlled clinical trial.
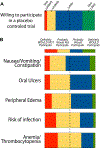
Results: A total of 317 and 558 participants responded to the first and second surveys, respectively. The majority of participants (77%) were willing to consider enrollment, regardless of having a 50% chance of receiving placebo versus treatment (78%). The majority (84%) of participants were willing to travel 200 miles to participate for up to six in-person visits over 50 days. Specific side effects, including anemia/thrombocytopenia (72%) or risk of infection (69.3%) had the greatest impact on clinical trial participation with other side effects (peripheral edema (53%), oral ulcers (51%), and gastrointestinal side effects (41%)) having less impact.
Conclusion: Patients with laryngotracheal stenosis possess nuanced insight into their disease and treatment options. As a group, they are extremely motivated for better therapies. Future laryngotracheal stenosis clinical trials should focus on providing excellent side effect -related education and utilizing feedback from online advocacy groups to optimize recruitment and retention.
Keywords: Laryngotracheal stenosis; clinical trial; cross-sectional survey; patient-guided trial design; placebo-controlled study; recruitment.
Conflict of interest statement
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.T.H. receives sponsored research agreement with Medtronic to investigate tracheostomy tube injury to the trachea.
Figures


References
-
- National Organization for Rare Disorders. Idiopathic Subglottic Stenosis NORD, https://rarediseases.org/rare-diseases/idiopathic-subglottic-stenosis (accessed 5 February 2021).
-
- Bell SA and Tudur Smith C. A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis 2014; 9(1): 170. - PMC - PubMed
-
- Kempf L, Goldsmith JC and Temple R. Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A 2018; 176(4): 773–783. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

