Anti-inflammatory role of GM1 and other gangliosides on microglia
- PMID: 34991625
- PMCID: PMC8739653
- DOI: 10.1186/s12974-021-02374-x
Anti-inflammatory role of GM1 and other gangliosides on microglia
Abstract
Background: Gangliosides are glycosphingolipids highly enriched in the brain, with important roles in cell signaling, cell-to-cell communication, and immunomodulation. Genetic defects in the ganglioside biosynthetic pathway result in severe neurodegenerative diseases, while a partial decrease in the levels of specific gangliosides was reported in Parkinson's disease and Huntington's disease. In models of both diseases and other conditions, administration of GM1-one of the most abundant gangliosides in the brain-provides neuroprotection. Most studies have focused on the direct neuroprotective effects of gangliosides on neurons, but their role in other brain cells, in particular microglia, is not known. In this study we investigated the effects of exogenous ganglioside administration and modulation of endogenous ganglioside levels on the response of microglia to inflammatory stimuli, which often contributes to initiation or exacerbation of neurodegeneration.
Methods: In vitro studies were performed using BV2 cells, mouse, rat, and human primary microglia cultures. Modulation of microglial ganglioside levels was achieved by administration of exogenous gangliosides, or by treatment with GENZ-123346 and L-t-PDMP, an inhibitor and an activator of glycolipid biosynthesis, respectively. Response of microglia to inflammatory stimuli (LPS, IL-1β, phagocytosis of latex beads) was measured by analysis of gene expression and/or secretion of pro-inflammatory cytokines. The effects of GM1 administration on microglia activation were also assessed in vivo in C57Bl/6 mice, following intraperitoneal injection of LPS.
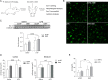
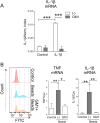
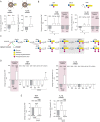
Results: GM1 decreased inflammatory microglia responses in vitro and in vivo, even when administered after microglia activation. These anti-inflammatory effects depended on the presence of the sialic acid residue in the GM1 glycan headgroup and the presence of a lipid tail. Other gangliosides shared similar anti-inflammatory effects in in vitro models, including GD3, GD1a, GD1b, and GT1b. Conversely, GM3 and GQ1b displayed pro-inflammatory activity. The anti-inflammatory effects of GM1 and other gangliosides were partially reproduced by increasing endogenous ganglioside levels with L-t-PDMP, whereas inhibition of glycolipid biosynthesis exacerbated microglial activation in response to LPS stimulation.
Conclusions: Our data suggest that gangliosides are important modulators of microglia inflammatory responses and reveal that administration of GM1 and other complex gangliosides exerts anti-inflammatory effects on microglia that could be exploited therapeutically.
Keywords: BV2 cells; GENZ-123346; GM1; Gangliosides; Inflammation; LPS; Liposomes; L–t-PDMP; Microglia.
© 2022. The Author(s).
Conflict of interest statement
SS and the University of Alberta hold a patent for the use of GM1 in HD. There are no other competing interests to declare.
Figures



Similar articles
-
Naïve Huntington's disease microglia mount a normal response to inflammatory stimuli but display a partially impaired development of innate immune tolerance that can be counteracted by ganglioside GM1.J Neuroinflammation. 2023 Nov 23;20(1):276. doi: 10.1186/s12974-023-02963-y. J Neuroinflammation. 2023. PMID: 37996924 Free PMC article.
-
GM1 induces p38 and microtubule dependent ramification of rat primary microglia in vitro.Brain Res. 2008 Dec 9;1244:13-23. doi: 10.1016/j.brainres.2008.09.072. Epub 2008 Oct 7. Brain Res. 2008. PMID: 18930716
-
Inhibition of human neuroblastoma cell proliferation and EGF receptor phosphorylation by gangliosides GM1, GM3, GD1A and GT1B.Cell Prolif. 2002 Apr;35(2):105-15. doi: 10.1046/j.1365-2184.2002.00228.x. Cell Prolif. 2002. PMID: 11952645 Free PMC article.
-
Gangliosides in nervous system development, regeneration, and pathologies.Neural Regen Res. 2023 Jan;18(1):81-86. doi: 10.4103/1673-5374.343890. Neural Regen Res. 2023. PMID: 35799513 Free PMC article. Review.
-
Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications.Front Neurosci. 2020 Oct 6;14:572965. doi: 10.3389/fnins.2020.572965. eCollection 2020. Front Neurosci. 2020. PMID: 33117120 Free PMC article. Review.
Cited by
-
Co-registration of MALDI-MSI and histology demonstrates gangliosides co-localize with amyloid beta plaques in Alzheimer's disease.Acta Neuropathol. 2024 Jun 19;147(1):105. doi: 10.1007/s00401-024-02759-1. Acta Neuropathol. 2024. PMID: 38896306 Free PMC article.
-
Ganglioside lipids inhibit the aggregation of the Alzheimer's amyloid-β peptide.RSC Chem Biol. 2025 Mar 13;6(5):809-822. doi: 10.1039/d4cb00189c. eCollection 2025 May 8. RSC Chem Biol. 2025. PMID: 40109301 Free PMC article.
-
Inhibition of USP30 Promotes Mitophagy by Regulating Ubiquitination of MFN2 by Parkin to Attenuate Early Brain Injury After SAH.Transl Stroke Res. 2025 Apr;16(2):448-466. doi: 10.1007/s12975-023-01228-3. Epub 2023 Dec 26. Transl Stroke Res. 2025. PMID: 38147294 Free PMC article.
-
Erythrocyte Plasma Membrane Lipid Composition Mirrors That of Neurons and Glial Cells in Murine Experimental In Vitro and In Vivo Inflammation.Cells. 2023 Feb 9;12(4):561. doi: 10.3390/cells12040561. Cells. 2023. PMID: 36831228 Free PMC article.
-
GM1 oligosaccharide efficacy against α-synuclein aggregation and toxicity in vitro.Biochim Biophys Acta Mol Cell Biol Lipids. 2023 Sep;1868(9):159350. doi: 10.1016/j.bbalip.2023.159350. Epub 2023 Jun 16. Biochim Biophys Acta Mol Cell Biol Lipids. 2023. PMID: 37330108 Free PMC article.
References
-
- Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–242. - PubMed
-
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. - PubMed
-
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16(5):543–551. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

