Nafamostat reduces systemic inflammation in TLR7-mediated virus-like illness
- PMID: 34991643
- PMCID: PMC8734544
- DOI: 10.1186/s12974-021-02357-y
Nafamostat reduces systemic inflammation in TLR7-mediated virus-like illness
Abstract
Background: The serine protease inhibitor nafamostat has been proposed as a treatment for COVID-19, by inhibiting TMPRSS2-mediated viral cell entry. Nafamostat has been shown to have other, immunomodulatory effects, which may be beneficial for treatment, however animal models of ssRNA virus infection are lacking. In this study, we examined the potential of the dual TLR7/8 agonist R848 to mimic the host response to an ssRNA virus infection and the associated behavioural response. In addition, we evaluated the anti-inflammatory effects of nafamostat in this model.
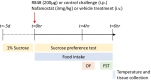
Methods: CD-1 mice received an intraperitoneal injection of R848 (200 μg, prepared in DMSO, diluted 1:10 in saline) or diluted DMSO alone, and an intravenous injection of either nafamostat (100 μL, 3 mg/kg in 5% dextrose) or 5% dextrose alone. Sickness behaviour was determined by temperature, food intake, sucrose preference test, open field and forced swim test. Blood and fresh liver, lung and brain were collected 6 h post-challenge to measure markers of peripheral and central inflammation by blood analysis, immunohistochemistry and qPCR.
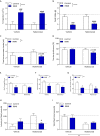
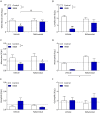
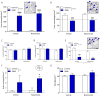
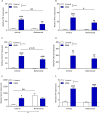
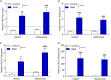
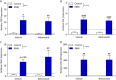
Results: R848 induced a robust inflammatory response, as evidenced by increased expression of TNF, IFN-γ, CXCL1 and CXCL10 in the liver, lung and brain, as well as a sickness behaviour phenotype. Exogenous administration of nafamostat suppressed the hepatic inflammatory response, significantly reducing TNF and IFN-γ expression, but had no effect on lung or brain cytokine production. R848 administration depleted circulating leukocytes, which was restored by nafamostat treatment.
Conclusions: Our data indicate that R848 administration provides a useful model of ssRNA virus infection, which induces inflammation in the periphery and CNS, and virus infection-like illness. In turn, we show that nafamostat has a systemic anti-inflammatory effect in the presence of the TLR7/8 agonist. Therefore, the results indicate that nafamostat has anti-inflammatory actions, beyond its ability to inhibit TMPRSS2, that might potentiate its anti-viral actions in pathologies such as COVID-19.
Keywords: COVID-19; Inflammation; Nafamostat; Sickness behaviour; Viral infection.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Yamamoto M, Kiso M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Takeda M, Kinoshita N, Ohmagari N, Gohda J, Semba K, et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12(6):629. - PMC - PubMed
-
- Yamamoto M, Matsuyama S, Li X, Takeda M, Kawaguchi Y, Inoue JI, Matsuda Z. Identification of nafamostat as a potent inhibitor of middle east respiratory syndrome coronavirus s protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60:6532–6539. - PMC - PubMed
-
- Liu Y, Li C, Wang J, Fang Y, Sun H, Tao X, Zhou XF, Liao H. Nafamostat mesilate improves neurological outcome and axonal regeneration after stroke in rats. Mol Neurobiol. 2017;54:4217–4231. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

