Impact of azithromycin mass drug administration on the antibiotic-resistant gut microbiome in children: a randomized, controlled trial
- PMID: 34991704
- PMCID: PMC8740015
- DOI: 10.1186/s13099-021-00478-6
Impact of azithromycin mass drug administration on the antibiotic-resistant gut microbiome in children: a randomized, controlled trial
Abstract
Background: Mass drug administration (MDA) with azithromycin is the primary strategy for global trachoma control efforts. Numerous studies have reported secondary effects of MDA with azithromycin, including reductions in childhood mortality, diarrhoeal disease and malaria. Most recently, the MORDOR clinical trial demonstrated that MDA led to an overall reduction in all-cause childhood mortality in targeted communities. There is however concern about the potential of increased antimicrobial resistance in treated communities. This study evaluated the impact of azithromycin MDA on the prevalence of gastrointestinal carriage of macrolide-resistant bacteria in communities within the MORDOR Malawi study, additionally profiling changes in the gut microbiome after treatment. For faecal metagenomics, 60 children were sampled prior to treatment and 122 children after four rounds of MDA, half receiving azithromycin and half placebo.
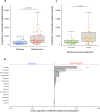
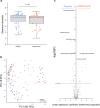
Results: The proportion of bacteria carrying macrolide resistance increased after azithromycin treatment. Diversity and global community structure of the gut was minimally impacted by treatment, however abundance of several species was altered by treatment. Notably, the putative human enteropathogen Escherichia albertii was more abundant after treatment.
Conclusions: MDA with azithromycin increased carriage of macrolide-resistant bacteria, but had limited impact on clinically relevant bacteria. However, increased abundance of enteropathogenic Escherichia species after treatment requires further, higher resolution investigation. Future studies should focus on the number of treatments and administration schedule to ensure clinical benefits continue to outweigh costs in antimicrobial resistance carriage. Trial registration ClinicalTrial.gov, NCT02047981. Registered January 29th 2014, https://clinicaltrials.gov/ct2/show/NCT02047981.
Keywords: Antimicrobial resistance; Azithromycin; Childhood mortality; Gut metagenomics; Gut microbiome; Macrolide resistance; Mass drug administration; Metagenomics; Microbial.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Whitty CJM, Glasgow KW, Sadiq ST, Mabey DC, Bailey R. Impact of community-based mass treatment for trachoma with oral azithromycin on general morbidity in Gambian children. Pediatr Infect Dis J. 1999;18:955–958. - PubMed
-
- Fry AM, Jha HC, Lietman TM, Chaudhary JSP, Bhatta RC, Elliott J, et al. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis. 2002;35:395–402. - PubMed
-
- Porco TC, Gebre T, Ayele B, House J, Keenan J, Zhou Z, et al. Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA. 2009;302:962–968. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

