Pembrolizumab plus azacitidine in patients with chemotherapy refractory metastatic colorectal cancer: a single-arm phase 2 trial and correlative biomarker analysis
- PMID: 34991708
- PMCID: PMC8740438
- DOI: 10.1186/s13148-021-01226-y
Pembrolizumab plus azacitidine in patients with chemotherapy refractory metastatic colorectal cancer: a single-arm phase 2 trial and correlative biomarker analysis
Abstract
Background: DNA mismatch repair proficient (pMMR) metastatic colorectal cancer (mCRC) is not responsive to pembrolizumab monotherapy. DNA methyltransferase inhibitors can promote antitumor immune responses. This clinical trial investigated whether concurrent treatment with azacitidine enhances the antitumor activity of pembrolizumab in mCRC.
Methods: We conducted a phase 2 single-arm trial evaluating activity and tolerability of pembrolizumab plus azacitidine in patients with chemotherapy-refractory mCRC (NCT02260440). Patients received pembrolizumab 200 mg IV on day 1 and azacitidine 100 mg SQ on days 1-5, every 3 weeks. A low fixed dose of azacitidine was chosen in order to reduce the possibility of a direct cytotoxic effect of the drug, since the main focus of this study was to investigate its potential immunomodulatory effect. The primary endpoint of this study was overall response rate (ORR) using RECIST v1.1., and secondary endpoints were progression-free survival (PFS) and overall survival (OS). Tumor tissue was collected pre- and on-treatment for correlative studies.
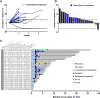
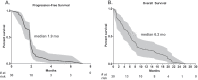
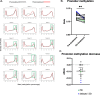
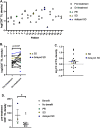
Results: Thirty chemotherapy-refractory patients received a median of three cycles of therapy. One patient achieved partial response (PR), and one patient had stable disease (SD) as best confirmed response. The ORR was 3%, median PFS was 1.9 months, and median OS was 6.3 months. The combination regimen was well-tolerated, and 96% of treatment-related adverse events (TRAEs) were grade 1/2. This trial was terminated prior to the accrual target of 40 patients due to lack of clinical efficacy. DNA methylation on-treatment as compared to pre-treatment decreased genome wide in 10 of 15 patients with paired biopsies and was significantly lower in gene promoter regions after treatment. These promoter demethylated genes represented a higher proportion of upregulated genes, including several immune gene sets, endogenous retroviral elements, and cancer-testis antigens. CD8+ TIL density trended higher on-treatment compared to pre-treatment. Higher CD8+ TIL density at baseline was associated with greater likelihood of benefit from treatment. On-treatment tumor demethylation correlated with the increases in tumor CD8+ TIL density.
Conclusions: The combination of pembrolizumab and azacitidine is safe and tolerable with modest clinical activity in the treatment for chemotherapy-refractory mCRC. Correlative studies suggest that tumor DNA demethylation and immunomodulation occurs. An association between tumor DNA demethylation and tumor-immune modulation suggests immune modulation and may result from treatment with azacitidine. Trial registration ClinicalTrials.gov, NCT02260440. Registered 9 October 2014, https://clinicaltrials.gov/ct2/show/NCT02260440 .
Keywords: Azacitidine; Colorectal cancer; DNA methyltransferase inhibitor; Epigenetic therapy; Immunotherapy; Mismatch repair proficient; PD-1; PD-L1; Pembrolizumab.
© 2022. The Author(s).
Conflict of interest statement
WS is the principal investigator of an investigator-initiated trial using pembrolizumab. NB serves on advisory boards for AstraZeneca, Bristol Meyer Squibb, Exelixis, and ThermoFisher. The remaining authors declare no potential conflicts of interest.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (London, England) 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

