The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas
- PMID: 34991965
- PMCID: PMC9250553
- DOI: 10.1016/j.preteyeres.2021.101039
The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas
Abstract
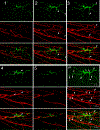
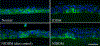
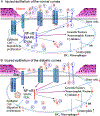
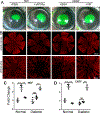
Diabetic peripheral neuropathy (DPN) is the most common complication of diabetes, with several underlying pathophysiological mechanisms, some of which are still uncertain. The cornea is an avascular tissue and sensitive to hyperglycemia, resulting in several diabetic corneal complications including delayed epithelial wound healing, recurrent erosions, neuropathy, loss of sensitivity, and tear film changes. The manifestation of DPN in the cornea is referred to as diabetic neurotrophic keratopathy (DNK). Recent studies have revealed that disturbed epithelial-neural-immune cell interactions are a major cause of DNK. The epithelium is supplied by a dense network of sensory nerve endings and dendritic cell processes, and it secretes growth/neurotrophic factors and cytokines to nourish these neighboring cells. In turn, sensory nerve endings release neuropeptides to suppress inflammation and promote epithelial wound healing, while resident immune cells provide neurotrophic and growth factors to support neuronal and epithelial cells, respectively. Diabetes greatly perturbs these interdependencies, resulting in suppressed epithelial proliferation, sensory neuropathy, and a decreased density of dendritic cells. Clinically, this results in a markedly delayed wound healing and impaired sensory nerve regeneration in response to insult and injury. Current treatments for DPN and DNK largely focus on managing the severe complications of the disease. Cell-based therapies hold promise for providing more effective treatment for diabetic keratopathy and corneal ulcers.
Keywords: Corneal wound healing; Diabetic keratopathy; Diabetic peripheral nerve degeneration.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Author Statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures











References
-
- Abdelkader H, Patel DV, McGhee C, Alany RG, 2011. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Experiment Ophthalmol 39, 259–270. - PubMed
-
- Adeghate E, Rashed H, Rajbandari S, Singh J, 2006. Pattern of distribution of calcitonin gene-related Peptide in the dorsal root ganglion of animal models of diabetes mellitus. Ann N Y Acad Sci 1084, 296–303. - PubMed

