Deconstructing Methanosarcina acetivorans into an acetogenic archaeon
- PMID: 34992140
- PMCID: PMC8764690
- DOI: 10.1073/pnas.2113853119
Deconstructing Methanosarcina acetivorans into an acetogenic archaeon
Abstract
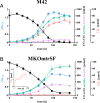
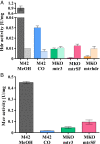
The reductive acetyl-coenzyme A (acetyl-CoA) pathway, whereby carbon dioxide is sequentially reduced to acetyl-CoA via coenzyme-bound C1 intermediates, is the only autotrophic pathway that can at the same time be the means for energy conservation. A conceptually similar metabolism and a key process in the global carbon cycle is methanogenesis, the biogenic formation of methane. All known methanogenic archaea depend on methanogenesis to sustain growth and use the reductive acetyl-CoA pathway for autotrophic carbon fixation. Here, we converted a methanogen into an acetogen and show that Methanosarcina acetivorans can dispense with methanogenesis for energy conservation completely. By targeted disruption of the methanogenic pathway, followed by adaptive evolution, a strain was created that sustained growth via carbon monoxide-dependent acetogenesis. A minute flux (less than 0.2% of the carbon monoxide consumed) through the methane-liberating reaction remained essential, indicating that currently living methanogens utilize metabolites of this reaction also for anabolic purposes. These results suggest that the metabolic flexibility of methanogenic archaea might be much greater than currently known. Also, our ability to deconstruct a methanogen into an acetogen by merely removing cellular functions provides experimental support for the notion that methanogenesis could have evolved from the reductive acetyl-coenzyme A pathway.
Keywords: Methanosarcina; acetogenic; acetyl-CoA pathway; methanogenic.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Huber C., Wächtershäuser G., Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science 276, 245–247 (1997). - PubMed
-
- Preiner M., et al. , A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 4, 534–542 (2020). - PubMed
-
- Ljungdahl L. G., The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu. Rev. Microbiol. 40, 415–450 (1986). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

