Targeted α-Emitter Therapy with 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial
- PMID: 34992153
- PMCID: PMC9454455
- DOI: 10.2967/jnumed.121.263230
Targeted α-Emitter Therapy with 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial
Abstract
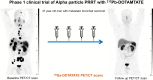
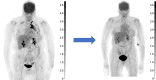
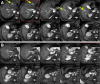
Peptide receptor radiotherapy with somatostatin analogs has been successfully used for years as a treatment for somatostatin-overexpressing tumors. Treatment of neuroendocrine tumors (NETs) with the β-particle emitter 177Lu-DOTATATE is currently considered the standard of care for subjects with gastroenteropancreatic NETs. Despite the success of 177Lu-DOTATATE, there remains significant room for improvement in terms of both safety and efficacy. Targeted α-emitter therapy with isotopes such as 212Pb has the potential to improve both. Here, we present the preliminary results of the phase 1 first-in-humans dose-escalation trial evaluating 212Pb-DOTAMTATE (a bifunctional metal chelator [DOTAM] and the SSTR-targeting peptide [TATE]) in patients with somatostatin receptor-positive NETs. Methods: Twenty subjects with histologically confirmed NETs, prior positive somatostatin analog scans, and no prior history of 177Lu/90Y/111In peptide receptor radiotherapy, with different primary sites of the disease, were enrolled. Treatment began with single ascending doses of 212Pb-DOTAMTATE, with subsequent cohorts receiving an incremental 30% dose increase, which was continued until a tumor response or a dose-limiting toxicity was observed. This was followed by a multiple ascending dose regimen. The recommended phase 2 dose regimen consisted of 4 cycles of 2.50 MBq/kg (67.6 μCi/kg) of 212Pb-DOTAMTATE administered at 8-wk intervals, intravenously. Results: Ten subjects received the highest dose, 2.50 MBq/kg/cycle (67.6 μCi/kg/cycle). Treatment was well tolerated, with the most common treatment-emergent adverse events being nausea, fatigue, and alopecia. No serious treatment-emergent adverse events were related to the study drug, and no subjects required treatment delay or a dose reduction. An objective radiologic response of 80% was observed for the first 10 subjects treated at the recommended phase 2 dose. Conclusion: Targeted α-therapy with 212Pb-DOTAMTATE has been shown to be well tolerated. Preliminary efficacy results are highly promising. If these results are confirmed in a larger, multicenter clinical trial, 212Pb-DOTAMTATE would provide a substantial benefit over currently Food and Drug Administration-approved therapies for patients with metastatic or inoperable SSTR-expressing NETs regardless of the grade and location of the primary tumor.
Keywords: 212Pb-DOTAMTATE; NEN; NET; PRRT; TAT; phase 1.
© 2022 by the Society of Nuclear Medicine and Molecular Imaging.
Figures


References
-
- Fani M, Nicolas GP, Wild D. Somatostatin receptor antagonists for imaging and therapy. J Nucl Med. 2017;58(suppl 2):61S–66S. - PubMed
-
- Shah MH, Goldner WS, Halfdanarson TR, et al. . NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018. J Natl Compr Canc Netw. 2018;16:693–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
