Large-vessel vasculitis
- PMID: 34992251
- PMCID: PMC9115766
- DOI: 10.1038/s41572-021-00327-5
Large-vessel vasculitis
Abstract
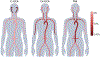
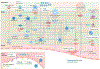
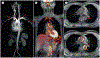
Large-vessel vasculitis (LVV) manifests as inflammation of the aorta and its major branches and is the most common primary vasculitis in adults. LVV comprises two distinct conditions, giant cell arteritis and Takayasu arteritis, although the phenotypic spectrum of primary LVV is complex. Non-specific symptoms often predominate and so patients with LVV present to a range of health-care providers and settings. Rapid diagnosis, specialist referral and early treatment are key to good patient outcomes. Unfortunately, disease relapse remains common and chronic vascular complications are a source of considerable morbidity. Although accurate monitoring of disease activity is challenging, progress in vascular imaging techniques and the measurement of laboratory biomarkers may facilitate better matching of treatment intensity with disease activity. Further, advances in our understanding of disease pathophysiology have paved the way for novel biologic treatments that target important mediators of disease in both giant cell arteritis and Takayasu arteritis. This work has highlighted the substantial heterogeneity present within LVV and the importance of an individualized therapeutic approach. Future work will focus on understanding the mechanisms of persisting vascular inflammation, which will inform the development of increasingly sophisticated imaging technologies. Together, these will enable better disease prognostication, limit treatment-associated adverse effects, and facilitate targeted development and use of novel therapies.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



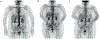

References
-
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K and Watts RA. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. - PubMed
-
- Horton BT, Magath TB and Brown GE. Arteritis of the temporal vessels: a previously undescribed form. Archives of internal medicine. 1934;53:400–409.
-
- Kogstad OA. Polymyalgia rheumatica and its relation to arteritis temporalis. Acta Med Scand. 1965;178:591–8. - PubMed
-
- Gilmour JR. Giant cell chronic arteritis. J Pathol Bacteriol. 1941;53:263–277.

