Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial
- PMID: 34992255
- PMCID: PMC9042909
- DOI: 10.1038/s41416-021-01644-y
Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial
Abstract
Background: Colorectal cancer (CRC) patients have a better prognosis if metastases are resectable. Initially, unresectable liver-only metastases can be converted to resectable with chemotherapy plus a targeted therapy. We assessed which of chemotherapy doublet (2-CTx) or triplet (3-CTx), combined with targeted therapy by RAS status, would be better in this setting.
Methods: PRODIGE 14 was an open-label, multicenter, randomised Phase 2 trial. CRC patients with initially defined unresectable liver-only metastases received either, 2-CTx (FOLFOX or FOLFIRI) or 3-CTx (FOLFIRINOX), plus bevacizumab/cetuximab by RAS status. The primary endpoint was to increase the R0/R1 liver-resection rate from 50 to 70% with the 3-CTx.
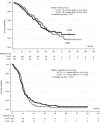
Results: Patients (n = 256) were mainly men with an ECOG PS of 0, and a median age of 60 years. In total, 109 patients (42.6%) had RAS-mutated tumours. After a median follow-up of 45.6 months, the R0/R1 liver-resection rate was 56.9% (95% CI: 48-66) with the 3-CTx versus 48.4% (95% CI: 39-57) with the 2-CTx (P = 0.17). Median overall survival was 43.4 months with 3-CTx versus 40 months with 2-CTx.
Conclusion: We failed to increase from 50 to 70% the R0/R1 liver-resection rate with the use of 3-CTx combined with bevacizumab or cetuximab by RAS status in CRC patients with initially unresectable liver metastases.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
MY reported receiving honoraria from Amgen, Bayer, Merck, Roche, and Servier. MR has nothing to disclose. ST has nothing to disclose. RG reported personal fees from AAA, Amgen, Astra-Zeneca, BMS, P. Fabre, Novartis, Roche, Servier, outside the submitted work. FG served on external advisory boards for Roche; research funding from Roche, Genentech, Amgen, Enterome, Servier; received funding for a clinical trial from Astra-Zeneca, received fee for communication by Amgen, Astra-Zeneca, BMS, Sanofi, Merck-Serono, Servier and received fee for travel by Roche and Servier. AM-B has nothing to disclose. LM has nothing to disclose. EF reported personal fees from ROCHE, personal fees from SERVIER, personal fees from NOVARTIS, personal fees from MSD, outside the submitted work. FK reported personal feed from Sanofi, other fees from Roche (congress support), other fees from Ipsen (congress support), outside the submitted work. MC has nothing to disclose. RK has nothing to disclose. MF has nothing to disclose. PH has nothing to disclose. TA reported personal fees from Roche, personal fees from Servier, personal fees from Amgen, personal fees from Ipsen, personal fees from Sanofi, non-financial support from Bayer, outside the submitted work. M-PG reported other relevant financial interest from Roche (travel), other relevant financial interest from Amgen (travel, board), outside the submitted work. FA reported personal fees from Sanofi, personal fees from Merck, personal fees from Amgen, personal fees from Servier, personal fees from Roche, outside the submitted work. EA reported other fees (advisory board) from Roche, from Astrazeneca, from Ipsen, from Bayer, from Sanofi, from AMGEN, from AAA, outside the submitted work. EL-C has nothing to disclose, CJ has nothing to disclose. AA reported receiving honoraria from Bayer, Bristol-Myers Squibb, Merck-Sharp Dohme, Sanofi, and Servier. RA reported personal fees from Merck (congress presentation), personal fees from Sanofi (congress presentation), outside the submitted work. OB reported personal fees from ROCHE (self honoraria, advisory/consultancy), personal fees from AMGEN (self honoraria, speaker bureau/expert testimony), personal fees from MERCK KGaA (self honoraria, advisory/consultancy), personal fees from SERVIER (self honoraria, speaker bureau/expert testimony), personal fees from BAYER (self honoraria, advisory/consultancy), personal fees from PIERRE FABRE (self honoraria, speaker bureau/expert testimony), personal fees from Astra-Zeneca (self honoraria, advisory/consultancy), personal fees from Grunenthal (self honoraria, advisory/consultancy), personal fees from MSD (Self honoraria, advisory/consultancy), non-financial support from Roche (travel/accommodation/expenses) and non-financial support from Servier (travel/accommodation/expenses) outside the submitted work.
Figures
References
-
- Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–8. doi: 10.1093/annonc/mdq714. - DOI - PubMed
-
- Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75. doi: 10.1016/S1470-2045(14)70330-4. - DOI - PubMed
-
- Gruenberger T, Bridgewater J, Chau I, Garcia Alfonso P, Rivoire M, Mudan S, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702–8. doi: 10.1093/annonc/mdu580. - DOI - PubMed
-
- Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. J Am Med Assoc. 2017;317:2392–2401. doi: 10.1001/jama.2017.7105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical