Integrative Bioinformatics Analysis Demonstrates the Prognostic Value of Chromatin Accessibility Biomarkers in Clear Cell Renal Cell Carcinoma
- PMID: 34993155
- PMCID: PMC8724435
- DOI: 10.3389/fonc.2021.814396
Integrative Bioinformatics Analysis Demonstrates the Prognostic Value of Chromatin Accessibility Biomarkers in Clear Cell Renal Cell Carcinoma
Abstract
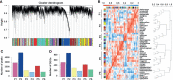
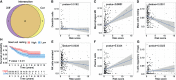
Clear cell renal cell carcinoma (ccRCC) accounts for 75%-85% of renal cell carcinoma (RCC) and has a poor 5-year survival rate. In recent years, medical advancement has promoted the understanding of the histopathological and molecular characterization of ccRCC; however, the carcinogenesis and molecular mechanisms of ccRCC remain unclear. Chromatin accessibility is an essential determinant of cellular phenotype. This study aimed to explore the potential role of chromatin accessibility in the development and progression of ccRCC. By the combination of open-access genome-wide chromatin accessibility profiles and gene expression profiles in ccRCC, we obtained a total of 13,474 crucial peaks, corresponding to 5,120 crucial genes and 9,185 differentially expressed genes. Moreover, two potential function modules (P2 and G4) that contained 129 upregulated genes were identified via the weighted gene co-expression network analysis (WGCNA). Furthermore, we obtained five independent predictors (FSCN1, SLC17A9, ANKRD13B, ADCY2, and MAPT), and a prognostic model was established based on these genes through the least absolute shrinkage and selection operator-proportional hazards model (LASSO-Cox) analysis. This model can stratify the ccRCC samples into a high-risk and a low-risk group, from which the patients have distinct prognosis. Further analysis demonstrated a completely different immune cell infiltration pattern between these two risk groups. This study also suggested that mast cell resting is associated with the prognosis of ccRCC and could be a target of immunotherapy. Overall, this study indicated that chromatin accessibility plays an essential role in ccRCC. The five prognostic chromatin accessibility biomarkers and the prognostic immune cells can provide a new direction for the treatment of ccRCC.
Keywords: carcinogenesis; chromatin; clear cell renal cell carcinoma; computational biology; gene expression profiling; weighted gene co-expression network analysis.
Copyright © 2021 Meng, Lan, Tian, Qin, Li, Li and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources
Miscellaneous

