Titanium Hydride Nanoplates Enable 5 wt% of Reversible Hydrogen Storage by Sodium Alanate below 80°C
- PMID: 34993488
- PMCID: PMC8696284
- DOI: 10.34133/2021/9819176
Titanium Hydride Nanoplates Enable 5 wt% of Reversible Hydrogen Storage by Sodium Alanate below 80°C
Abstract
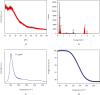
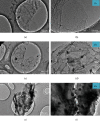
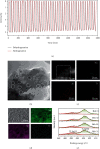
Sodium alanate (NaAlH4) with 5.6 wt% of hydrogen capacity suffers seriously from the sluggish kinetics for reversible hydrogen storage. Ti-based dopants such as TiCl4, TiCl3, TiF3, and TiO2 are prominent in enhancing the dehydrogenation kinetics and hence reducing the operation temperature. The tradeoff, however, is a considerable decrease of the reversible hydrogen capacity, which largely lowers the practical value of NaAlH4. Here, we successfully synthesized a new Ti-dopant, i.e., TiH2 as nanoplates with ~50 nm in lateral size and ~15 nm in thickness by an ultrasound-driven metathesis reaction between TiCl4 and LiH in THF with graphene as supports (denoted as NP-TiH2@G). Doping of 7 wt% NP-TiH2@G enables a full dehydrogenation of NaAlH4 at 80°C and rehydrogenation at 30°C under 100 atm H2 with a reversible hydrogen capacity of 5 wt%, superior to all literature results reported so far. This indicates that nanostructured TiH2 is much more effective than Ti-dopants in improving the hydrogen storage performance of NaAlH4. Our finding not only pushes the practical application of NaAlH4 forward greatly but also opens up new opportunities to tailor the kinetics with the minimal capacity loss.
Copyright © 2021 Zhuanghe Ren et al.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Lang C. G., Jia Y., Yao X. Recent advances in liquid-phase chemical hydrogen storage. Energy Storage Materials . 2020;26:290–312. doi: 10.1016/j.ensm.2020.01.010. - DOI
-
- He T., Pachfule P., Wu H., Xu Q., Chen P. Hydrogen carriers. Nature Reviews Materials . 2016;1(12, article 16067) doi: 10.1038/natrevmats.2016.59. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

