Bone marrow NG2+/Nestin+ mesenchymal stem cells drive DTC dormancy via TGFβ2
- PMID: 34993493
- PMCID: PMC8730384
- DOI: 10.1038/s43018-021-00179-8
Bone marrow NG2+/Nestin+ mesenchymal stem cells drive DTC dormancy via TGFβ2
Abstract
In the bone marrow (BM) microenvironment, where breast cancer (BC) disseminated tumour cells (DTCs) can remain dormant for decades, NG2+/Nestin+ mesenchymal stem cells (MSCs) promote hematopoietic stem cell quiescence. Here, we reveal that periarteriolar BM-resident NG2+/Nestin+ MSCs can also instruct BC DTCs to enter dormancy. NG2+/Nestin+ MSCs produce TGFβ2 and BMP7 and activate a quiescence pathway dependent on TGFBRIII and BMPRII, which via p38-kinase result in p27 induction. Genetic depletion of MSCs or conditional knock-out of TGFβ2 in MSCs using an NG2-CreER driver led to bone metastatic outgrowth of otherwise dormant p27+/Ki67- DTCs. Also ER+ BC patients without systemic recurrence displayed higher frequency of TGFβ2 and BMP7 detection in the BM. Our results provide a direct proof that HSC dormancy niches control BC DTC dormancy and suggest that aging or extrinsic factors that affect the NG2+/Nestin+ MSC niche homeostasis may result in a break from dormancy and BC bone relapse.
Figures




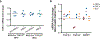
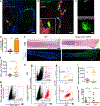
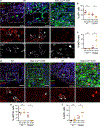

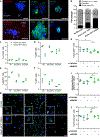


References
-
- Chaffer CL & Weinberg RA A perspective on cancer cell metastasis. Science vol. 331 1559–1564 (2011). - PubMed
-
- Sherry MM, Greco FA, Johnson DH & Hainsworth JD Metastatic breast cancer confined to the skeletal system. An indolent disease. Am. J. Med. 81, 381–386 (1986). - PubMed
-
- Engel J et al. The process of metastasisation for breast cancer. Eur. J. Cancer 39, 1794–1806 (2003). - PubMed
-
- H??semann Y. et al. Systemic Spread Is an Early Step in Breast Cancer. Cancer Cell 13, 58–68 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA244780/CA/NCI NIH HHS/United States
- R01 DK112976/DK/NIDDK NIH HHS/United States
- R01 CA218024/CA/NCI NIH HHS/United States
- R01 HL069438/HL/NHLBI NIH HHS/United States
- R01 CA218578/CA/NCI NIH HHS/United States
- R01 CA216248/CA/NCI NIH HHS/United States
- R01 CA109182/CA/NCI NIH HHS/United States
- U01 DK116312/DK/NIDDK NIH HHS/United States
- R01 HL145064/HL/NHLBI NIH HHS/United States
- R01 HL126705/HL/NHLBI NIH HHS/United States
- K22 CA196750/CA/NCI NIH HHS/United States
- R01 DK056638/DK/NIDDK NIH HHS/United States
- P30 CA196521/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

