Loss of p19Arf promotes fibroblast survival during leucine deprivation
- PMID: 34994382
- PMCID: PMC8864297
- DOI: 10.1242/bio.058728
Loss of p19Arf promotes fibroblast survival during leucine deprivation
Abstract
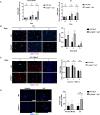
Fibroblasts are quiescent and tumor suppressive in nature but become activated in wound healing and cancer. The response of fibroblasts to cellular stress has not been extensively investigated, however the p53 tumor suppressor has been shown to be activated in fibroblasts during nutrient deprivation. Since the p19 Alternative reading frame (p19Arf) tumor suppressor is a key regulator of p53 activation during oncogenic stress, we investigated the role of p19Arf in fibroblasts during nutrient deprivation. Here, we show that prolonged leucine deprivation results in increased expression and nuclear localization of p19Arf, triggering apoptosis in primary murine adult lung fibroblasts (ALFs). In contrast, the absence of p19Arf during long-term leucine deprivation resulted in increased ALF proliferation, migration and survival through upregulation of the Integrated Stress Response pathway and increased autophagic flux. Our data implicates a new role for p19Arf in response to nutrient deprivation. This article has an associated First Person interview with the first author of the paper.
Keywords: P19Arf; Autophagy; Fibroblast; Integrated stress response; Leucine deprivation.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Arandkar, S., Furth, N., Elisha, Y., Nataraj, N. B., van der Kuip, H., Yarden, Y., Aulitzky, W., Ulitsky, I., Geiger, B. and Oren, M. (2018). Altered p53 functionality in cancer-associated fibroblasts contributes to their cancer-supporting features. Proc. Natl. Acad. Sci. U.S.A., 115, 6410-6415. 10.1073/pnas.1719076115 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

