Common Ancestry-Specific Ion Channel Variants Predispose to Drug-Induced Arrhythmias
- PMID: 34994586
- PMCID: PMC8852297
- DOI: 10.1161/CIRCULATIONAHA.121.054883
Common Ancestry-Specific Ion Channel Variants Predispose to Drug-Induced Arrhythmias
Abstract
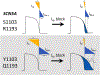
Background: Multiple reports associate the cardiac sodium channel gene (SCN5A) variants S1103Y and R1193Q with type 3 congenital long QT syndrome and drug-induced long QT syndrome. These variants are too common in ancestral populations to be highly arrhythmogenic at baseline, however: S1103Y allele frequency is 8.1% in African Americans and R1193Q 6.1% in East Asians. R1193Q is known to increase late sodium current (INa-L) in cardiomyocytes derived from induced pluripotent stem cells but the role of these variants in modulating repolarization remains poorly understood.
Methods: We determined the effect of S1103Y on QT intervals among African-American participants in a large electronic health record. Using cardiomyocytes derived from induced pluripotent stem cells carrying naturally occurring or genome-edited variants, we studied action potential durations (APDs) at baseline and after challenge with the repolarizing potassium current (IKr) blocker dofetilide and INa-L and IKr at baseline.
Results: In 1479 African-American participants with no confounding medications or diagnoses of heart disease, QT intervals in S1103Y carriers was no different from that in noncarriers. Baseline APD was no different in cells expressing the Y allele (SY, YY cells) compared with isogenic cells with the reference allele (SS cells). However, INa-L was increased in SY and YY cells and the INa-L blocker GS967 shortened APD in SY/YY but not SS cells (P<0.001). IKr was increased almost 2-fold in SY/YY cells compared with SS cells (tail current: 0.66±0.1 versus 1.2±0.1 pA/pF; P<0.001). Dofetilide challenge prolonged APD at much lower concentrations in SY (4.1 nmol/L [interquartile range, 1.5-9.3]; n=11) and YY (4.2 nmol/L [1.7-5.0]; n=5) than in SS cells (249 nmol/L [22.3-2905]; n=14; P<0.001 and P<0.01, respectively) and elicited afterdepolarizations in 8/16 SY/YY cells but only in 1/14 SS cells. R1193Q cells similarly displayed no difference in baseline APD but increased IKr and increased dofetilide sensitivity.
Conclusions: These common ancestry-specific variants do not affect baseline repolarization, despite generating increased INa-L. We propose that increased IKr serves to maintain normal repolarization but increases the risk of manifest QT prolongation with IKr block in variant carriers. Our findings emphasize the need for inclusion of diverse populations in the study of adverse drug reactions.
Keywords: Torsades de Pointes; drug-related side effects and adverse reactions; induced pluripotent stem cells; long QT syndrome; myocytes, cardiac; potassium channels; sodium channels.
Conflict of interest statement
Figures
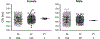


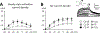
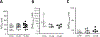
References
-
- Paulussen AD, Gilissen RA, Armstrong M, Doevendans PA, Verhasselt P, Smeets HJ, Schulze-Bahr E, Haverkamp W, Breithardt G, Cohen N, et al. Genetic variations of kcnq1, kcnh2, scn5a, kcne1, and kcne2 in drug-induced long qt syndrome patients. Journal of molecular medicine (Berlin, Germany). 2004;82:182–188 - PubMed
-
- Weeke P, Mosley JD, Hanna D, Delaney JT, Shaffer C, Wells QS, Van Driest S, Karnes JH, Ingram C, Guo Y, et al. Exome sequencing implicates an increased burden of rare potassium channel variants in the risk of drug-induced long qt interval syndrome. Journal of the American College of Cardiology. 2014;63:1430–1437 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

