Circulating tumor DNA for prognosis assessment and postoperative management after curative-intent resection of colorectal liver metastases
- PMID: 34994972
- PMCID: PMC9303459
- DOI: 10.1002/ijc.33924
Circulating tumor DNA for prognosis assessment and postoperative management after curative-intent resection of colorectal liver metastases
Abstract
The recurrence rate of colorectal liver metastases (CRLM) patients treated with curative intent is above 50%. Standard of care surveillance includes intensive computed tomographic (CT) imaging as well as carcinoembryonic antigen (CEA) measurements. Nonetheless, relapse detection often happens too late to resume curative treatment. This longitudinal cohort study enrolled 115 patients with plasma samples (N = 439) prospectively collected before surgery, postoperatively at day 30 and every third month for up to 3 years. Droplet digital PCR (ddPCR) was used to monitor serial plasma samples for somatic mutations. Assessment of ctDNA status either immediately after surgery, or serially during surveillance, stratified the patients into groups of high and low recurrence risk (hazard ratio [HR], 7.6; 95% CI, 3.0-19.7; P < .0001; and HR, 4.3; 95% CI, 2.3-8.1; P < .0001, respectively). The positive predictive value (PPV) of ctDNA was 100% in all postoperative analyses. In multivariable analyses, postoperative ctDNA status was the only consistently significant risk marker associated with relapse (P < .0001). Indeterminate CT findings were observed for 30.8% (21/68) of patients. All patients (9/21) that were ctDNA positive at the time of the indeterminate CT scan later relapsed, contrasting 42.6% (5/12) of those ctDNA negative (P = .0046). Recurrence diagnoses in patients with indeterminate CT findings were delayed (median 2.8 months, P < .0001). ctDNA status is strongly associated with detection of minimal residual disease and early detection of relapse. Furthermore, ctDNA status can potentially contribute to clinical decision-making in case of indeterminate CT findings, reducing time-to-intervention.
Keywords: circulating tumor DNA; colorectal cancer liver metastases; droplet digital PCR; minimal residual disease; recurrence surveillance.
© 2022 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
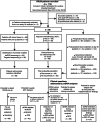
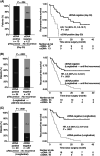
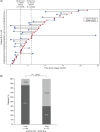
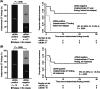
References
-
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941‐1953. - PubMed
-
- Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271‐1280. - PubMed
-
- Eadens MJ, Grothey A. Curable metastatic colorectal cancer. Curr Oncol Rep. 2011;13:168‐176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

