Targeted therapy for advanced anaplastic lymphoma kinase (<I>ALK</I>)-rearranged non-small cell lung cancer
- PMID: 34994987
- PMCID: PMC8740884
- DOI: 10.1002/14651858.CD013453.pub2
Targeted therapy for advanced anaplastic lymphoma kinase (<I>ALK</I>)-rearranged non-small cell lung cancer
Abstract
Background: Targeted therapies directed at specific driver oncogenes have improved outcomes for individuals with advanced non-small cell lung cancer (NSCLC). Approximately 5% of lung adenocarcinomas, the most common histologic subtype of NSCLC, harbour rearrangements in the anaplastic lymphoma kinase (ALK) gene leading to constitutive activity of the ALK kinase. Crizotinib was the first tyrosine kinase inhibitor (TKI) demonstrated to be effective in advanced NSCLC. Next-generation ALK TKIs have since been developed including ceritinib, alectinib, brigatinib, ensartinib, and lorlatinib, and have been compared with crizotinib or chemotherapy in randomised controlled trials (RCTs). These ALK-targeted therapies are currently used in clinical practice and are endorsed in multiple clinical oncology guidelines.
Objectives: To evaluate the safety and efficacy of ALK inhibitors given as monotherapy to treat advanced ALK-rearranged NSCLC.
Search methods: We conducted electronic searches in the Cochrane Lung Cancer Group Specialised Register, Cochrane Central Register of Controlled Trials, MEDLINE, and Embase. We also searched conference proceedings from the American Society for Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), and International Association for the Study of Lung Cancer (IASLC) World Conference on Lung Cancer, as well as the reference lists of retrieved articles. All searches were conducted from 2007 until 7 January 2021.
Selection criteria: We included RCTs comparing ALK inhibitors with cytotoxic chemotherapy or another ALK inhibitor in individuals with incurable locally advanced or metastatic pathologically confirmed ALK-rearranged NSCLC.
Data collection and analysis: Two review authors independently assessed studies for eligibility, extracted study characteristics and outcome data, and assessed risk of bias using the Cochrane risk of bias tool for each included study. We assessed the certainty of evidence using GRADE. Primary outcomes were progression-free survival (PFS) and adverse events (AE); secondary outcomes were overall survival (OS), OS at one year, overall response rate (ORR) by RECIST (Response Evaluation Criteria in Solid Tumours) criteria, and health-related quality of life (HRQoL). We performed a meta-analysis for all outcomes, where appropriate, using the fixed-effect model. We reported hazard ratios (HR) for PFS, OS, and a composite HRQoL of life outcome (time to deterioration), and risk ratios (RR) for AE, ORR, and one-year OS. We presented 95% confidence intervals (95% CIs) and used the I² statistic to investigate heterogeneity. We planned comparisons of 'ALK inhibitor versus chemotherapy' and 'next-generation ALK inhibitor versus crizotinib' with subgroup analysis by type of ALK inhibitor, line of treatment, and baseline central nervous system involvement.
Main results: Eleven studies (2874 participants) met our inclusion criteria: six studies compared an ALK inhibitor (crizotinib, ceritinib, and alectinib) to chemotherapy, and five studies compared a next-generation ALK inhibitor (alectinib, brigatinib, and lorlatinib) to crizotinib. We assessed the evidence for most outcomes as of moderate to high certainty. Most studies were at low risk for selection, attrition, and reporting bias; however, no RCTs were blinded, resulting in a high risk of performance and detection bias for outcomes reliant on subjective measurement. ALK inhibitor versus chemotherapy Treatment with ALK inhibitors resulted in a large increase in PFS compared to chemotherapy (HR 0.45, 95% CI 0.40 to 0.52, 6 RCTs, 1611 participants, high-certainty evidence). This was found regardless of line of treatment. ALK inhibitors may result in no difference in overall AE rate when compared to chemotherapy (RR 1.01, 95% CI 1.00 to 1.03, 5 RCTs, 1404 participants, low-certainty evidence). ALK inhibitors slightly improved OS (HR 0.84, 95% CI 0.72 to 0.97, 6 RCTs, 1611 participants, high-certainty evidence), despite most included studies having a significant number of participants crossing over from chemotherapy to receive an ALK inhibitor after the study period. ALK inhibitors likely increase ORR (RR 2.43, 95% CI 2.16 to 2.75, 6 RCTs, 1611 participants, moderate-certainty evidence) including in measurable baseline brain metastases (RR 4.88, 95% CI 2.18 to 10.95, 3 RCTs, 108 participants) when compared to chemotherapy. ALK inhibitors result in a large increase in the HRQoL measure, time to deterioration (HR 0.52, 95% CI 0.44 to 0.60, 5 RCTs, 1504 participants, high-certainty evidence) when compared to chemotherapy. Next-generation ALK inhibitor versus crizotinib Next-generation ALK inhibitors resulted in a large increase in PFS (HR 0.39, 95% CI 0.33 to 0.46, 5 RCTs, 1263 participants, high-certainty evidence), particularly in participants with baseline brain metastases. Next-generation ALK inhibitors likely result in no difference in overall AE (RR 1.00, 95% CI 0.98 to 1.01, 5 RCTs, 1263 participants, moderate-certainty evidence) when compared to crizotinib. Next-generation ALK inhibitors likely increase OS (HR 0.71, 95% CI 0.56 to 0.90, 5 RCTs, 1263 participants, moderate-certainty evidence) and slightly increase ORR (RR 1.18, 95% CI 1.10 to 1.25, 5 RCTs, 1229 participants, moderate-certainty evidence) including a response in measurable brain metastases (RR 2.45, 95% CI 1.7 to 3.54, 4 RCTs, 138 participants) when compared to crizotinib. Studies comparing ALK inhibitors were conducted exclusively or partly in the first-line setting.
Authors' conclusions: Next-generation ALK inhibitors including alectinib, brigatinib, and lorlatinib are the preferred first systemic treatment for individuals with advanced ALK-rearranged NSCLC. Further trials are ongoing including investigation of first-line ensartinib. Next-generation inhibitors have not been compared to each other, and it is unknown which should be used first and what subsequent treatment sequence is optimal.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Laird B Cameron: none known. LC is a member of the Thoracic Oncology Group Australasia (formerly Australasian Lung Cancer Trials Group) and chairperson of the New Zealand Lung Oncology Special Interest Group.
Nadia Hitchen: none known.
Elias Chandran: none known.
Tessa Morris: none known
Renée Manser: none known.
Benjamin J Solomon is an author on phase III clinical studies of crizotinib, ceritinib, and lorlatinib. He has served on advisory boards for Pfizer, Novartis, Roche‐Genentech, AstraZeneca, Merck, Bristol Myers Squibb, Gritstone Oncology, and Loxo Oncology, and he has spoken at meetings where costs were sponsored by industry.
Vanessa Jordan: none known.
Figures
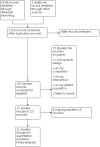
















































Update of
- doi: 10.1002/14651858.CD013453
References
References to studies included in this review
ALESIA 2019 {published data only}
-
- NCT02838420.A study to evaluate and compare the efficacy and safety of alectinib versus crizotinib and to evaluate the pharmacokinetics of alectinib in Asian participants with treatment-naive anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC). clinicaltrials.gov/ct2/show/NCT02838420.
-
- Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, et al.Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet 2019;7(5):437‐46. [DOI: 10.1016/S2213-2600(19)30053-0] - DOI - PubMed
-
- Zhou C, Lu Y, Kim S-W, Reungwetwattana T, Zhou J, Zhang Y, et al.Primary results of ALESIA: a randomised, phase III, open-label study of alectinib vs crizotinib in Asian patients with treatment-naive ALK1 advanced NSCLC. Annals of Oncology 2018;29:viii740. [DOI: 10.1093/annonc/mdy424.062] - DOI
-
- Zhou C, Lu Y, Kim S-W, Reungwetwattana T, Zhou J, Zhang Y, et al.Primary results of ALESIA: phase III, randomised open label study of alectinib (ALC) vs crizotinib (CRZ) in Asian patients (pts) with treatment-naive ALK+ advanced non-small-cell lung cancer (NSCLC). Annals of Oncology 2018;29(9):IX 174. [DOI: 10.1093/annonc/mdy483.001] - DOI
ALEX 2017 {published data only}
-
- Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al.Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non–small cell lung cancer in the global phase III ALEX study. Journal of Thoracic Oncology 2019;14(7):1233-43. [DOI: 10.1016/j.jtho.2019.03.007] - DOI - PubMed
-
- Camidge DR, Peters S, Mok T, Gadgeel SM, Cheema PK, Pavlakis N, et al.Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK+ NSCLC. Journal of Clinical Oncology 2018;36(15 Suppl):9043. [DOI: 10.1200/JCO.2018.36.15-suppl.9043] - DOI
-
- Dziadziuszko R, Mok TS, Camidge DR, Shaw AT, Noe J, Nowicka M, et al.Impact of the EML4-ALK variant on the efficacy of alectinib (ALC) in untreated ALK1 advanced NSCLC (aNSCLC) in the global phase III ALEX study. Annals of Oncology 2018;29:viii494‐5. [DOI: 10.1093/annonc/mdy292.002] - DOI
-
- Dziadziuszko R, Peters S, Mok T S K, Camidge D R, Noé J, Nowicka M, et al.Circulating free DNA as a prognostic biomarker in patients with advanced ALK+ NSCLC treated with alectinib from the global phase III ALEX trial. Journal of Clinical Oncology 2019;37(15 Suppl):9053.
-
- EUCTR2013-004133-33-PL.Alectinib versus crizotinib in previously untreated patients with ALK-positive non-small cell lung cancer. trialsearch.who.int/Trial2.aspx?TrialID=NCT02075840 27/02/2014.
ALTA‐1L 2019 {published data only}
-
- Ahn MJ, Kim HR, Yang JCH, Han JY, Lee JS, Hochmair MJ, et al.Brigatinib (BRG) versus crizotinib (CRZ) in Asian versus non-Asian patients (pts) in the phase III ALTA-1L trial. Journal of Clinical Oncology 2019;37(15 Suppl):9026. [DOI: 10.1200/JCO.2019.37.15_suppl.9026] - DOI
-
- Ahn M J, Kim H R, Yang J C H, Han J Y, Li J Y C, Hochmair MJ, et al.Brigatinib (BRG) versus crizotinib (CRZ) in Asian vs non-Asian patients (pts): update from ALTA-1L. Annals of Oncology 2020;31:S843-4.
-
- Califano R, Camidge D, Kim HR, Ahn MJ, Yang J, Han J, et al.Brigatinib versus crizotinib in patients with ALK inhibitor naive advanced ALK+ NSCLC: results from the phase 3 ALTA-1L trial. Lung Cancer 2020;139:S59‐60. [DOI: 10.1016/S0169-5002(20)30164-1] - DOI
-
- Califano R, Hochmair MJ, Gridelli C, Delmonte A, Garcia Campelo MR, Bearz A, et al.Brigatinib (BRG) vs crizotinib (CRZ) in the phase III ALTA-1L trial. Annals of Oncology 2019;30:ii41. [DOI: 10.1093/annonc/mdz063.004] - DOI
-
- Camidge D R, Kim HR, Ahn MJ, Yang JCH, HanJY, Hochmair MJ, et al.Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. Journal of Clinical Oncology 2020;38(31):3592-603. [DOI: 10.1200/JCO.20.00505] - DOI - PMC - PubMed
ALUR 2018 {published data only}
-
- De Castro J, Novello S, Mazieres J, Oh IJ, Migliorino MR, Helland A, et al.CNS efficacy results from the phase III ALUR study of alectinib versus chemotherapy in previously treated ALK1 NSCLC. Annals of Oncology 2017;28(Suppl 5):v481. [DOI: 10.1093/annonc/mdx380.048] - DOI
-
- EUCTR2015-000634-29-SK.A study of alectinib versus pemetrexed or docetaxel in patients with anaplastic lymphoma kinase-positive advanced non small cell lung cancer. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2015-000634-29-PT.
-
- Mazieres J, Novello S, De Castro J, Migliorino MR, Helland Å, Dziadziuszko R, et al.P1.01-013 Patient-reported outcomes and safety from the phase III ALUR study of alectinib versus chemotherapy in pre-treated ALK+ NSCLC. Journal of Thoracic Oncology 2017;12(11):S1897. [DOI: 10.1016/j.jtho.2017.09.667] - DOI
-
- Mazieres J, Novello S, De Castro J, Migliorino MR, Helland A, Dziadziuszko R, et al.Patient-reported outcomes and safety from the phase III ALUR study of alectinib versus chemotherapy in pre-treated ALK+ NSCLC. Journal of Thoracic Oncology 2017;12(11):S1897.
-
- NCT02604342.Alectinib versus pemetrexed or docetaxel in anaplastic lymphoma kinase (ALK)-Positive advanced non-small cell lung cancer (NSCLC) participants previously treated with platinum-based chemotherapy and crizotinib. clinicaltrials.gov/ct2/show/NCT02604342.
ASCEND‐4 2017 {published data only}
-
- De Castro G, Shao-Weng Tan D, Crino L, Wu YL, Paz-Ares L, Wolf J, et al.First-line ceritinib versus chemotherapy in patients with ALK-rearranged (ALK+) NSCLC: a randomized, phase 3 study (ASCEND-4). Journal of Thoracic Oncology 2017;12(1):S7.
-
- Erratum: department of Error (The Lancet (2017) 389(10072) (917-929) (S014067361730123X) (10.1016/S0140-6736(17)30123-X)). Lancet 2017;389(10072):917-29. [DOI: 10.1016/S0140-6736(17)30123-X] - DOI
-
- NCT01828099.LDK378 versus chemotherapy in previously untreated patients with ALK rearranged non-small cell lung cancer. clinicaltrials.gov/ct2/show/NCT01828099.
-
- Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al.First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389(10072):917-29. [DOI: 10.1016/S0140-6736%2817%2930123-X] - DOI - PubMed
-
- Tan DS, Geater S, Yu CJ, Tsai CM, Hsia TC, Zhou J, et al.First-line ceritinib versus chemotherapy in patients (pts) with advanced ALK rearranged (ALK1) non-small cell lung cancer (NSCLC): ASCEND-4 Asian subgroup analysis. Annals of Oncology 2019;30:v599‐600. [DOI: 10.1093/annonc/mdz259.016] - DOI
ASCEND‐5 2017 {published data only}
-
- Ceritinib outperforms chemo as second-line treatment. Cancer Discovery 2016;6(12):OF5. [DOI: 10.1158/2159-8290.CD-NB2016-135] - DOI - PubMed
-
- Kiura K, Imamura F, Kagamu H, Matsumoto S, Hida T, Nakagawa K, et al.Phase 3 study of ceritinib versus chemotherapy in ALK-rearranged NSCLC patients previously treated with chemotherapy and crizotinib (ASCEND-5): Japanese subset. Japanese Journal of Clinical Oncology 2018;48(4):367‐75. [DOI: 10.1093/jjco/hyy016] - DOI - PMC - PubMed
-
- Mok TSK, Scagliotti G, Kim TM, Crino L, Liu G, Gridelli C, et al.Patient-reported outcomes (PROs) in ASCEND-5: a randomized, phase 3 study of ceritinib versus chemotherapy (CT) in patients (pts) with advanced anaplastic lymphoma kinase rearranged (ALK+) NSCLC previously treated with CT and crizotinib (CRZ). Annals of Oncology 2016;27:ix142‐3. [DOI: 10.1093/annonc/mdw594.008] - DOI
-
- NCT01828112.LDK378 versus chemotherapy in ALK rearranged (ALK positive) patients previously treated with chemotherapy (platinum doublet) and crizotinib. clinicaltrials.gov/ct2/show/NCT01828112.
CROWN 2020 {published data only}
-
- CTRI/2018/01/011325.Study with investigational drug PF-06463922 and comparator crizotinib in patients with a specific type of advanced lung cancer. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=22321&EncHid....
-
- EUCTR2016-003315-35-PL.Study with investigational drug PF-06463922 and comparator crizotinib in patients with a specific type of advanced lung cancer. clinicaltrialsregister.eu/ctr-search/search?query=2016-003315-35.
-
- Lorlatinib outperforms crizotinib in NSCLC. Cancer Discovery 2021;11(1):OF5. [DOI: 10.1158/2159-8290.CD-NB2020-110] - DOI - PubMed
-
- NCT03052608.A study of lorlatinib versus crizotinib in first line treatment of patients with ALK-positive NSCLC. clinicaltrials.gov/ct2/show/NCT03052608.
-
- Shaw A, Bauer T, Takahashi T, Baik C, Goto Y, Polli A, et al.First-line lorlatinib versus crizotinib for advanced anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer. Journal of Thoracic Oncology 2018;13(10):S584. [DOI: 10.1016/j.jtho.2018.08.863] - DOI
J‐ALEX 2017 {published data only}
-
- Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al.Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390(10089):29‐39. [DOI: 10.1016/S0140-6736(17)30565-2] - PubMed
-
- Hsu JC, Jaminion F, Guerini E, Tanaka T, Golding S, Balas B, et al.Population pharmacokinetic and exposure-efficacy/safety analyses for bridging J-ALEX to global population with alectinib 600 mg BID dose regimen. Journal of Clinical Oncology 2017;35(15 Suppl):e20616. [DOI: 10.1007/s10928-017-9536-y] - DOI
-
- JPRN-JapicCTI-132316.Open-label randomized phase III study of the efficacy and safety of CH5424802(AF802) in ALK-positive advanced or recurrent non-small cell lung cancer with crizotinib control. www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-132316.
-
- Kim Y, Hida T, Nokihara H, Kondo M, Azuma K, Seto T, et al.Alectinib (ALC) versus crizotinib (CRZ) in ALK-positive non-small cell lung cancer (ALK+ NSCLC): primary results from phase III study (J-ALEX). Journal of Thoracic Oncology 2017;12(1):S378‐9.
-
- Nakagawa K, Hida T, Nokihara H, Morise M, Azuma K, Kim YH, et al.Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2020;139:195-9. - PubMed
PROFILE 1007 2013 {published data only}
-
- Blackhall F, Hirsh V, Kim DW, Besse B, Nokihara H, Han JY, et al .Impact of crizotinib on patient-reported general health status compared with single-agent chemotherapy in a phase III study of advanced ALK-positive non-small cell lung cancer (NSCLC). European Journal of Cancer 2013;49(Suppl 2):S799‐800. [DOI: 10.1016/S0959-8049%2813%2970065-0] - DOI
-
- Blackhall F, Kim DW, Besse B, Nokihara H, Han JY, Wilner KD, et al.Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. Journal of Thoracic Oncology 2014;9(11):1625‐33. [DOI: 10.1097/JTO.0000000000000318] - DOI - PubMed
-
- Erratum: patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. Journal of Thoracic Oncology 2015;10(11):1657. [DOI: 10.1097/JTO.0000000000000702] - PubMed
-
- EUCTR2009-012595-27-BG.Phase 3, randomized, open-label study of the efficacy and safety of pf 02341066 versus standard of care chemotherapy (pemetrexed or docetaxel) in patients with advanced non-small cell lung cancer (Nsclc) harboring a translocation or inversion event involving the anaplastic lymphoma kinase (Alk) gene locus. trialsearch.who.int/Trial2.aspx?TrialID=NCT00932893 (first received 30 June 2009).
-
- NCT00932893.An investigational drug, PF-02341066 is being studied versus standard of care in patients with advanced non-small cell lung cancer with a specific gene profile involving the anaplastic lymphoma kinase (ALK) gene. clinicaltrials.gov/ct2/show/NCT00932893.
PROFILE 1014 2014 {published data only}
-
- EUCTR2010-021336-33-PT.Cancer of the lung with special characteristics (nonsquamous histology and ALK fusion gene event). trialsearch.who.int/Trial2.aspx?TrialID=NCT01154140.
-
- Felip E, Blackhall FH, Mok T, Cappuzzo F, Wilner KD, Reisman A, et al.Impact of crizotinib on patient-reported general health status compared with chemotherapy in patients with no prior systemic treatment for advanced non-squamous ALK-positive non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2015;33(15 Suppl):8101.
-
- Lu Y, Zhou J, Chung CH, Masters E, Wilner K, Selaru P, et al.Patient-reported symptoms and quality of life (QOL) in East Asian patients with ALK+ NSCLC treated with crizotinib versus chemotherapy. Journal of Thoracic Oncology 2017;12(1):S1167.
-
- Mok TSK, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, et al.Overall survival (OS) for first-line crizotinib versus chemotherapy in ALK1 lung cancer: updated results from PROFILE 1014. Annals of Oncology 2017;28(Suppl 5):v605-49. [DOI: 10.1093/annonc/mdx440.053] - DOI
-
- Nakagawa K, Kim DW, Wu YL, Solomon BJ, Mekhail T, Felip E, et al.First-line crizotinib versus pemetrexed + cisplatin/carboplatin in Asian patients with advanced ALK+ NSCLC in PROFILE 1014. Annals of Oncology 2014;25(Suppl 5):v2. [DOI: 10.1093/annonc/mdu395.1] - DOI
PROFILE 1029 2018 {published data only}
-
- NCT01639001.A study of crizotinib versus chemotherapy in previously untreated ALK positive East Asian non-small cell lung cancer patients. clinicaltrials.gov/ct2/show/NCT01639001.
-
- Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al.Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non–small cell lung cancer. Journal of Thoracic Oncology 2018;13(10):1539‐48. [DOI: 10.1016/j.jtho.2018.06.012] - DOI - PubMed
-
- Zhou J, Lu Y, Chung CH, Masters E, Wilner K, Tang Y, Wu YL.Patient reported general health status in a study of crizotinib versus chemotherapy in patients with non-small cell lung cancer (NSCLC). Journal of Thoracic Oncology 2017;12(1):S1169.
References to studies excluded from this review
Blackhall 2017 {published data only}
-
- Blackhall F, Ross Camidge D, Shaw AT, Soria JC, Solomon BJ, et al.Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open 2017;2(3):e000219. [DOI: 10.1136/esmoopen-2017-000219] - DOI - PMC - PubMed
Cho 2017 {published data only}
-
- Cho BC, Kim DW, Bearz A, Laurie SA, McKeage M, Borra G, et al.ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). Journal of Thoracic Oncology 2017;12(9):1357‐67. [DOI: 10.1016/j.jtho.2017.07.005] - DOI - PubMed
-
- Cho BC, Obermannova R, Bearz A, Kim D, Orlov S, Borra G, et al.Efficacy and updated safety of ceritinib (450 mg or 600 mg) with low-fat meal vs 750 mg fasted in ALK+ metastatic NSCLC. Journal of Thoracic Oncology 2017;12(11):S1757.
-
- Cho BC, Obermannova R, Bearz A, McKeage M, Kim DW, Batra U, et al.Efficacy and safety of ceritinib (450 mg/d or 600 mg/d) with food versus 750-mg/d fasted in patients with ALK receptor tyrosine kinase (ALK)–positive NSCLC: primary efficacy results from the ASCEND-8 study. Journal of Thoracic Oncology 2019;14(7):1255-65. [DOI: 10.1016/j.jtho.2019.03.002] - DOI - PubMed
Chow 2019 {published data only}
-
- Chow LQ, Barlesi F, Bertino EM, den Bent MJ, Wakelee H, Wen PY, et al.Results of the ASCEND-7 phase II study evaluating ALK inhibitor (ALKi) ceritinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) metastatic to the brain. Annals of Oncology 2019;30(Suppl 5):v602‐3.
EUCTR2012‐003474‐36‐BE {published data only}
-
- EUCTR2012-003474-36-BE.A clinical study of oral LDK378 in adult patients with ALK-activated non-small cell lung cancer and who have not been previously treated with crizotinib. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (first received 25 October 2012).
Felip 2016 {published data only}
-
- Felip E, Orlov S, Park K, Yu C, Tsai CM, Nishio M, et al.Phase 2 study of ceritinib in ALKi-naive patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC): whole body responses in the overall pt group and in pts with baseline brain metastases (BM). In: Annals of Oncology. Vol. 27. 2016. [DOI: 10.1093/annonc/mdw383.3] - DOI
Gao 2016 {published data only}
-
- Gao L, Zhang Y, Hu B, Liu J, Kong P, Lou S, et al.Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord–derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. Journal of Clinical Oncology 2016;34(24):2843-50. - PubMed
JPRN‐JapicCTI‐184073 {published data only}
-
- JPRN-JapicCTI-184073.A study comparing adjuvant alectinib versus adjuvant platinum-based chemotherapy in patients with ALK positive non-small cell lung cancer. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-JapicCTI-184073.
Kim 2016 {published data only}
-
- Ahn M, Camidge DR, Tiseo M, Reckamp K, Hansen K, Kim S, et al.Brigatinib in crizotinib-refractory ALK+ NSCLC: updated efficacy and safety results from ALTA, a randomized phase 2 trial. Journal of Thoracic Oncology 2017;12(11):S1755‐6.
-
- Ahn MJ, Camidge DR, Tiseo M, Reckamp KL, Hansen KH, Kim SW, et al.Brigatinib (BRG) in crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): updates from ALTA, a pivotal randomized phase 2 trial. Journal of Clinical Oncology 2017;35(15 Suppl):e20503.
-
- Camidge DR, Kim DW, Tiseo M, Langer CJ, Ahn MJ, Shaw AT, et al.Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. Journal of Clinical Oncology 2018;36(26):2693‐701. [DOI: 10.1200/JCO.2017.77.5841] - DOI - PubMed
-
- Camidge DR, Tiseo M, Ahn M, Reckamp K, Hansen K, Kim S, et al.Depth of target lesion response to brigatinib and its association with outcomes in patients with ALK+ NSCLC in the ALTA trial. Journal of Thoracic Oncology 2017;12(11):S1892.
-
- Camidge DR, Tiseo M, Ahn MJ, Reckamp K, Hansen K, Kim SW, et al.Brigatinib in crizotinib-refractory ALK+ NSCLC: central assessment and updates from ALTA, a pivotal randomized phase 2 trial. Journal of Thoracic Oncology 2017;12(1):S1167‐9.
Lenderking 2017 {published data only}
-
- Lenderking WR, Speck RM, Huang JT, Huang H, Kerstein D, Reichmann W, et al.Evaluating clinically meaningful change of the EORTC QLQ-C30 in patients with NSCLC. Value in Health 2017;20(5):A120.
Liang 2019 {published data only}
-
- Liang F, Shen D.EP1.14-02 comparative efficacy of first-line ceritinib at a dose of 450mg with food and alectinib in advanced ALK+ NSCLC. Journal of Thoracic Oncology 2019;14(10):S1032. [DOI: 10.1016/j.jtho.2019.08.2287] - DOI
NCT02134912 {published data only}
-
- NCT02134912.S1300: Pemetrexed Disodium With or Without Crizotinib in Treating Patients With Stage IV Non-Small Cell Lung Cancer That Has Progressed After Crizotinib. clinicaltrials.gov/ct2/show/NCT02134912.
Park 2020 {published data only}
-
- Park S, Lee YG, Park JH, Lee GW, Kang EJ, Choi YJ, et al.A phase III, open-label, randomized study of atezolizumab in combination with carboplatin + paclitaxel + bevacizumab compared with pemetrexed + cisplatin or carboplatin with stage IV non-squamous non-small cell lung cancer (NSCLC) with activating EGFR mutation or ALK translocation (ATLAS Trial). Journal of Clinical Oncology 2020;38(15 Suppl):TPS9636.
Reckamp 2019 {published data only}
-
- Reckamp K, Lin HM, Huang J, Proskorovsky I, Reichmann W, Krotneva S, et al.Comparative efficacy of brigatinib versus ceritinib and alectinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small cell lung cancer. Current Medicine Respiratory Opinion 2019;35(4):569-76. [DOI: 10.1080/03007995.2018.1520696] - DOI - PubMed
Wolf 2015 {published data only}
-
- Wolf J, Schneider CP, Potzner M, Cazorla Arratia P, Shen J, Branle F, et al.The phase II ASCEND-7 (CLDK378A2205) trial: ceritinib in patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) metastatic to the brain and/or leptomeninges. Oncology Research and Treatment 2015;38:138. [DOI: 10.1159/000439070] - DOI
References to ongoing studies
eXalt3 2020 {published data only}
-
- EUCTR2015-004147-40-NL.A clinical trial comparing ensartinib (study drug) to crizotinib in lung cancer patients. trialsearch.who.int/Trial2.aspx?TrialID=NCT02767804.
-
- Horn L, Wu Y, Reck M, Wakelee H, Liang C, Harrow K, et al.eXalt3: phase 3 randomized study comparing ensartinib to crizotinib in anaplastic lymphoma kinase positive non-small cell lung cancer patients. Journal of Thoracic Oncology 2018;13(10):S582. [DOI: 10.1016/j.jtho.2018.08.858] - DOI
-
- Horn L, Wu YL, Reck M, Liang C, Tan F, Harrow K, et al.EXalt3: a phase III study of ensartinib (X-396) in anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2017;35(15):TPS8578.
-
- Horn L, Wu YL, Reck M, Liang C, Tan F, Harrow K, et al.EXalt3: phase 3 randomized study comparing ensartinib to crizotinib in anaplastic lymphoma kinase (ALK) positive non-small cell lung cancer (NSCLC) patients. Journal of Thoracic Oncology 2018;13(4):S116.
-
- Horn L, Wu YL, Reck M, Liang C, Tan F, Harrow K et al.EXalt3: a phase III study of ensartinib (X-396) in anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). Annals of Oncology 2017;28:iii51. [DOI: 10.1093/annonc/mdx085] - DOI
NCT03737994 {published data only}
-
- NCT03737994.Biomarker/ALK inhibitor combinations in treating patients with stage IV ALK positive non-small cell lung cancer (The NCI-NRG ALK master protocol). clinicaltrials.gov/ct2/show/NCT03737994.
NCT04009317 {published data only}
-
- NCT04009317.Study of TQ-B3139 versus crizotinib in the first line treatment of subjects with anaplastic lymphoma kinase (ALK) positive non-small cell lung cancer (NSCLC). clinicaltrials.gov/ct2/show/NCT04009317.
NCT04318938 {published data only}
-
- NCT04318938.Advancing brigatinib properties in ALK+ NSCLC patients by deep phenotyping. clinicaltrials.gov/ct2/show/NCT04318938.
NCT04632758 {published data only}
-
- NCT04632758.Study comparing WX-0593 to crizotinib in ALK positive non-small cell lung cancer (NSCLC) patients. clinicaltrials.gov/ct2/show/NCT04632758.
Popat 2019 {published data only}
-
- NCT03596866.An efficacy study comparing brigatinib versus alectinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer participants who have progressed on crizotinib. clinicaltrials.gov/ct2/show/NCT03596866.
-
- Popat S, Liu G, Lu S, Song G, Samnotra V, Yang JCH.Phase III ALTA-3 study of brigatinib (BRG) versus alectinib (ALC) in patients (pts) with advanced anaplastic lymphoma kinase (ALK)−positive non–small cell lung cancer (NSCLC) that progressed on crizotinib (CRZ). Annals of Oncology 2019;30:v653-4. [DOI: 10.1093/annonc/mdz260.108] - DOI
Additional references
Aaronson 1993
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al.The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85:365-76. - PubMed
Anota 2015
-
- Anota A, Hamidou Z, Paget-Bailly S, Chibaudel B, Bascoul-Mollevi C, Auquier P, et al.Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Quality Life Research 2015;24(1):5-18. - PMC - PubMed
ASCO guideline
-
- Hanna NH, Robinson AG, Temin S, Baker S Jr, Brahmer JR, Ellis PM, et al.Therapy for stage IV non–small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. Journal of Clinical Oncology 2021;39(9):1040-91. - PubMed
Barlesi 2016
-
- Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, et al.Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French cooperative thoracic intergroup (IFCT). Lancet 2016;387(10026):1415-26. - PubMed
Barrows 2019
Breadner 2020
-
- Breadner D, Blanchette P, Shanmuganathan S, Boldt RG, Raphael J.Efficacy and safety of ALK inhibitors in ALK-rearranged non-small cell lung cancer: a systematic review and meta-analysis. Lung Cancer 2020;144:57-63. - PubMed
Cameron 2015
-
- Cameron L, Solomon B.New treatment options for ALK-rearranged non-small cell lung cancer. Current Treatment Options in Oncology 2015;16(10):49. [1534-6277: (Electronic)] - PubMed
Camidge 2012
Cetin 2011
Chansky 2017
-
- Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallieres E, Groome P, et al.The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. Journal of Thoracic Oncology 2017;12(7):1109-21. - PubMed
Chuang 2021
Costa 2011
-
- Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al.CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. Journal of Clinical Oncology 2011;29(15):e443-5. - PubMed
Costa 2015
Costa 2018
Covidence [Computer program]
-
- Covidence.Version accessed 20 September 2017. Melbourne, Australia: Veritas Health Innovation, 2014. Available at covidence.org.
Creelan 2019
-
- Creelan BC, Yeh T, Kim S-W, Nogami N, Kim D-W, Chow LQ, et al.Phase I study of gefitinib (G) + durvalumab (D) for locally advanced/metastatic non-small cell lung cancer (NSCLC) harbouring epidermal growth factor receptor (EGFR) sensitising mutations. Annals of Oncology 2019;30(Suppl 2):ii31-7.
CTCAE v4
-
- US Department of Health and Human Services, National Institutes of Health, National Cancer Institute.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published: May 28, 2009 (v4.03: June 14, 2010). www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Eisenhauer 2009
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al.New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228-47. - PubMed
Elliott 2020
ESMO guideline
Fukuoka 2011
-
- Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al.Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). Journal of Clinical Oncology 2011;29(21):2866-74. - PubMed
Gainor 2015
Gainor 2016
Gainor 2016a
Goldstraw 2016
-
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al.The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. Journal of Thoracic Oncology 2016;11(1):39-51. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT: GRADEpro Guideline Development Tool.Version accessed 20 September 2017. McMaster University and Evidence Prime, 2021. Available at gradepro.org.
Guyatt 2008
Higgins 2011
-
- Higgins JP, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/. [1119964792]
Hirsch 2016
-
- Hirsch FR, Suda K, Wiens J, Bunn PA Jr.New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016;388(10048):1012-24. - PubMed
Kalemkerian 2018
-
- Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al.Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. Journal of Clinical Oncology 2018;36(9):911. - PubMed
Kassem 2019
-
- Kassem L, Shohdy KS, Lasheen S, Abdel-Rahman O, Ali A, Abdel-Malek RR.Safety issues with the ALK inhibitors in the treatment of NSCLC: a systematic review. Critical Review Oncology Hematology 2019;134:56-64. - PubMed
Khan 2019
Kim 2018
Koller 2017
-
- Koller M, Hjermstad MJ, Tomaszewski KA, Tomaszewska IM, Hornslien K, Harle A, et al on behalf of the EORTC .Quality of Life Group, EORTC Lung Cancer Group, and European Society of Thoracic Surgeons. An international study to revise the EORTC questionnaire for assessing quality of life in lung cancer patients. Annals of Oncology 2017;28(11):2874-81. [DOI: 10.1093/annonc/mdx453.] - DOI - PubMed
Kris 2014
Lin 2017
Mazieres 2019
McDermott 2008
-
- McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, et al.Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Research 2008;68(9):3389-95. - PubMed
Moher 2009
Moya‐Horno 2018
Murray 2021
NCT04849273
-
- NCT04849273.A Study of TPX-0131, a Novel Oral ALK Tyrosine Kinase Inhibitor, in Patients With ALK+ Advanced or Metastatic NSCLC. clinicaltrials.gov/ct2/show/NCT04849273.
Novello 2016
-
- Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al.Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2016;27(Suppl 5):v1-27. [1569-8041: (Electronic)] - PubMed
Pacheco 2019
Pacheco 2019a
Planchard 2018
-
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al.Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2018;29(Suppl 4):iv192-237. - PubMed
Reck 2021
Recondo 2018
-
- Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L.Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? National Review of Clinical Oncology 2018;15(11):694-708. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rikova 2007
-
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al.Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131(6):1190-203. [0092-8674: (Print)] - PubMed
Scagliotti 2008
-
- Scagliotti GV, Parikh P, Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al.Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. Journal of Clinical Oncology 2008;26(21):3543-51. [1527-7755: (Electronic)] - PubMed
Schiller 2002
-
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al.Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. New England Journal of Medicine 2002;346(2):92-8. [0028-4793] - PubMed
Schulz 2002
-
- Schulz KF, Grimes DA.Generation of allocation sequences in randomised trials: chance, not choice. Lancet 2002;359(9305):515-9. - PubMed
Seike 2018
-
- Seike M, Inoue A, Sugawara S, Morita S, Hosomi Y, Ikeda S, et al.Phase III study of gefitinib (G) versus gefitinib+carboplatin+pemetrexed (GCP) as first-line treatment for patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). Annals of Oncology 2018;29(Suppl 8):viii496. [DOI: 10.1093/annonc/mdy292.005] - DOI
Shaw 2009
Shaw 2013
-
- Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al.Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. New England Journal of Medicine 2013;368(25):2385-94. [1533-4406: (Electronic)] - PubMed
Shaw 2017
-
- Shaw AT, Peters S, Mok T, Gadgeel SM, Ahn JS, Ou S-HI, et al.Alectinib versus crizotinib in treatment-naive advanced ALK-positive non-small cell lung cancer (NSCLC): primary results of the global phase III ALEX study. ASCO Annual Meeting 2017. [0732-183X]
Siegel 2019
-
- Siegel RL, Miller KD, Jemal A.Cancer statistics, 2019. CA: a Cancer Journal for Clinicians 2019;69(1):7-34. - PubMed
Soda 2007
-
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al.Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448(7153):561-6. - PubMed
Soda 2008
Solomon 2009
-
- Solomon B, Varella-Garcia M, Camidge DR.ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. Journal of Thoracic Oncology 2009;4(12):1450-4. [1556-1380: (Electronic)] - PubMed
Solomon 2014
-
- Solomon BJ, Mok T, Kim D-W, Wu Y-L, Nakagawa K, Mekhail T, et al.First-line crizotinib versus chemotherapy in ALK-positive lung cancer. New England Journal of Medicine 2014;371(23):2167-77. [0028-4793] - PubMed
Solomon 2018
-
- Solomon BJ, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, et al.Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. Journal of Clinical Oncology 2018;36(22):2251-8. - PubMed
Soria 2017
-
- Soria J-C, Tan DS, Chiari R, Wu Y-L, Paz-Ares L, Wolf J, et al.First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389(10072):917-29. [0140-6736] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

