Patient-Reported Outcome-Based Symptom Management Versus Usual Care After Lung Cancer Surgery: A Multicenter Randomized Controlled Trial
- PMID: 34995100
- PMCID: PMC8937008
- DOI: 10.1200/JCO.21.01344
Patient-Reported Outcome-Based Symptom Management Versus Usual Care After Lung Cancer Surgery: A Multicenter Randomized Controlled Trial
Abstract
Purpose: We aimed to evaluate the efficacy and feasibility of patient-reported outcome (PRO)-based symptom management in the early period after lung cancer surgery.
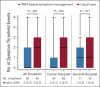
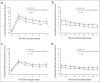
Methods: Before surgery, patients with clinically diagnosed lung cancer were randomly assigned 1:1 to receive postoperative PRO-based symptom management or usual care. All patients reported symptoms on MD Anderson Symptom Inventory-Lung Cancer presurgery, daily postsurgery, and twice a week after discharge for up to 4 weeks via an electronic PRO system. In the intervention group, treating surgeons responded to overthreshold electronic alerts driven by any of the five target symptom scores (score ≥ 4 on a 0-10 scale for pain, fatigue, disturbed sleep, shortness of breath, and coughing). The control group patients received usual care and no alerts were generated. The primary outcome was the number of symptom threshold events (any target symptom with a score of ≥ 4) at discharge. Per-protocol analyses were conducted.
Results: Of the 166 participants, 83 were randomly allocated to each group. At discharge, the intervention group reported fewer symptom threshold events than the control group (median [interquartile range], 0 [0-2] v 2 [0-3]; P = .007). At 4 weeks postdischarge, this difference was maintained between the intervention and control groups (median [interquartile range], 0 [0-0] v 0 [0-1]; P = .018). The intervention group had a lower complication rate than the control group (21.5% v 40.6%; P = .019). Surgeons spent a median of 3 minutes managing an alert.
Conclusion: PRO-based symptom management after lung cancer surgery showed lower symptom burden and fewer complications than usual care for up to 4 weeks postdischarge.
Conflict of interest statement
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 - PubMed
-
- Lin S, Chen Y, Yang L, et al. : Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs 22:1281-1290, 2013 - PubMed
-
- Di Maio M, Gallo C, Leighl NB, et al. : Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J Clin Oncol 33:910-915, 2015 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

